The Food and Drug Administration approved the first copy of a biotechnology drug for the U.S. market, firing the starting gun on a new industry that could help the U.S. curb its $376 billion in yearly drug spending.
The drug is a rival version of Neupogen, an Amgen Inc. treatment prescribed to chemotherapy patients. The copycat medicine, Zarxio from Novartis AG , is the first approved under a new regulatory framework designed to introduce competition for costly biotech drugs, which are produced in living cells and typically administered by infusion or injection.
Lower-priced copies of biotech drugs are estimated to save the U.S. $47 billion over the next 10 years, according to consulting firm Avalere Health LLC. Unlike regular pills, biotechnology drugs haven’t faced generic competition when their patents expire because they are much harder to copy, and because regulators until now couldn’t figure out how to approve knockoffs that were highly similar but not exact replicas. The knockoffs are known as “biosimilars.”
Biosimilars of some of the world’s most expensive drugs could hit the U.S. market in the next few years, from the arthritis treatments Humira and Remicade to the breast cancer drug Herceptin. But their arrival comes with a host of unknowns, including how much less they will cost.
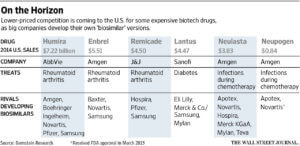
The following chart from the Wall Street Journal shows some of the major competitor drugs that have been developed. It remains to be seen how widely U.S. doctors will embrace biosimilars, or how fiercely branded drug makers will attempt to protect the original brands and possibly undermine biosimilar competitors.
Read full, original article: U.S. Clears First Biotech Copycat Drug, Jolting Sector