In part 1, we learned that avian flu is killing birds globally in record numbers, has crossed the species barrier to animals, and could pose a global threat to humans from a COVID-like virus if unchecked. Advances in CRISPR gene editing might lead to the development of genetically engineered poultry that would be resistant to the deadly H1N1 flu virus, protecting birds and mammals. But unscientific regulatory barriers are blocking its potential. If approved by regulators, the new strain would be one of only a handful of genetically engineered food animals in the world, compared to scores of important genetically engineered drugs.
Unfortunately, that’s unlikely to happen soon, and perhaps never at all. Rather than facilitating innovation in the ag biotech sector, under regulations currently in place, the US Food and Administration has all but blocked new products from going onto line. Compare that dismal record with, most notably, Japan.
In 2021, Japan approved the sale of the first CRISPR animals, genetically enhanced versions of red sea bream and a tiger puffer fish. The fish were genome edited to knock out a protein (myostatin) that suppresses muscle growth. Other gene-edited animals are being developed in various countries where regulations allow.

What’s the hold up in the US?
The glacial pace of the U.S. FDA’s approval process for genetically engineered food animals has inhibited innovation for decades. It wasn’t until 2021 that the first of them — the AquAdvantage Atlantic salmon tweaked using transgenic engineering (which makes it a “GMO”) to grow twice as quickly as the unaltered variety — finally went on sale in the US. The FDA review of the fish took more than 20 years. (Compare that to the five-month review of human insulin, the first biopharmaceutical, which was approved in 1982.)
Regulators have also approved two gene-edited animals for possible human consumption, but neither has yet been commercialized. The GalSafe pig, approved in 2020, was developed for people allergic to red meat, although it has yet to be marketed. And in 2022, FDA determined that beef cattle gene-edited to be born with a “slick coat” that helps them withstand hot weather was “low risk as a food product,” opening them up to further R&D. Regulators have also approved two other animals that were never commercialized — a genetically-modified goat, greenlighted in 2009, that produced a drug in its milk for preventing blood clots; and several other genetically altered animals have been approved to provide medical products.
Several fluorescent, colored zebra “GloFish” were approved and commercialized two decades ago, but they are more of a novelty for home aquariums than any sort of breakthrough.
Hardly a great track record of promoting innovation.
And don’t expect the situation will change soon when it comes to exploiting the potential of gene-editing to protect birds and other wildlife from H5N1 flu. We concluded Part 1 noting that the ability to confer resistance to avian flu could curtail infections and the massive culling of our poultry flocks, while at the same time substantially lowering the risk of transmission to wild bird populations, other mammals, and humans. But publishing in the scientific literature promising – or even revolutionary – results is a long way from these remarkable genetic innovations providing benefits in the real world.
Plants vs animals: US genetic engineering regulatory policy diverges dramatically
Unlike plants, which are regulated mostly by the US Department of Agriculture, genetically engineered animals fall under the jurisdiction of the FDA. The agency has exerted control over “genetically altered” agricultural animals, irrespective of risk, for decades. In the government’s logic-defying rationale, because engineering the DNA of animals changes the way a body works, genetically-engineered animals, either through cisgenic gene editing or transgenic genetic modification, are regulated as if they are animal drugs.
It is hard to see a compelling reason for this bizarre reasoning other than turf-building by the FDA. The 1986 Coordinated Framework for the Regulation of Biotechnology was supposed to guide the approval process of all genetically engineered plants and animals under the jurisdiction of three agencies: the FDA, USDA and the U.S. Environmental Protection Agency (EPA). Initially, the Coordinated Framework designated the USDA as the responsible agency and called for regulation of only “unreasonable” risks. But the FDA claimed jurisdiction.
To justify its control of the approval process of genetically engineered animals, the FDA expansively interpreted its statutory responsibilities for regulating animal drugs. The agency declared that genetically altered DNA in an animal is equivalent to administering a chemical intended to change the animal’s “structure and function,” and thus fits the legal definition of a “drug.”
Technically, the FDA is “not treating gene-edited animals as drugs,” said Alta Charo, a lawyer and bioethicist at the University of Wisconsin Law School. “They … regulate the altered DNA as a drug” based on the 1938 Food, Drug, and Cosmetic Act, which defines a drug as something that intentionally alters a body’s structure or function.”
But that makes no scientific sense; we call that explanation “lawyering without logic.” Animal scientists and breeders have a long history of safely crafting important genetic improvements in animals, from livestock to companion animals — using imprecise, pre-genetic engineering techniques — without government involvement. It’s called animal breeding.
Will CRISPR push the FDA towards regulatory reform?
While the gene-editing revolution offered the government an opportunity to reassess its current bizarre regulatory structure, nothing has come of it. Using its “enforcement discretion,” the agency claims that for the new generation of gene-edited animals, it will make “low-risk determinations” based on what it claims are more flexible standards, and will allow some genetically altered animals to be marketed without formal approval. However, even that ‘informal’ process will be lengthy, arbitrary, and expensive for researchers and corporations hoping to commercialize this expensive technology, exacerbating the uncertainty.
The current system creates large opportunity costs amounting to billions of dollars. Many genetically altered farm animals have been developed, raised to adulthood through several generations, with the research then abandoned because the costs of bringing them to market were too high.
The FDA’s approval process is also opaque, even to other government agencies. The agency has never reported the number of genetically-altered animals that it has under review, whether that number has changed over time, or the percentages that have been approved or rejected.
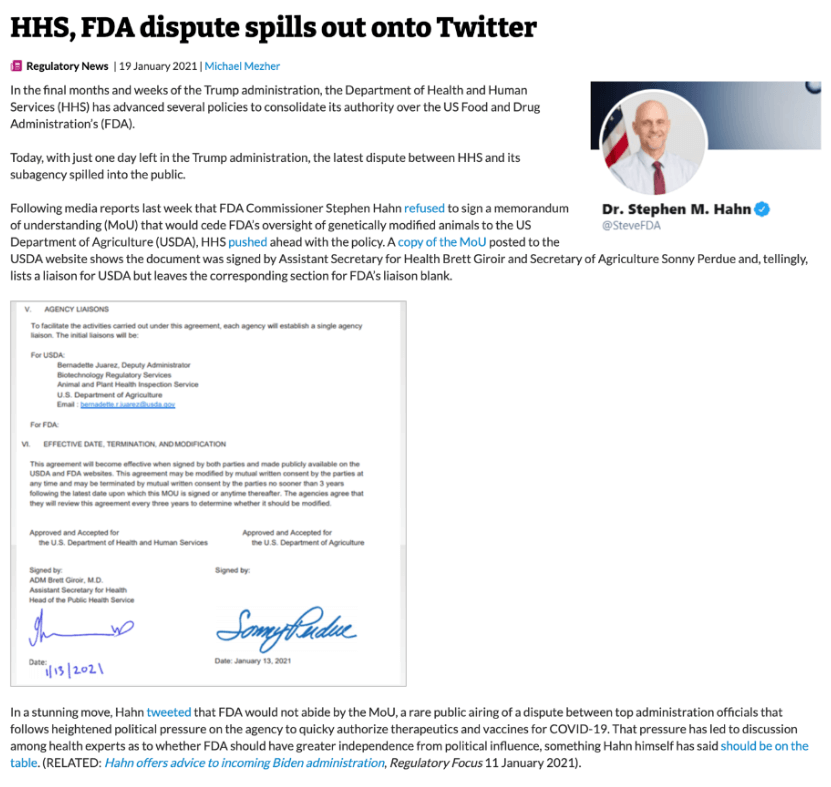
One possibility for improving the regulatory climate would be for FDA to relinquish oversight to the far more flexible Department of Agriculture. Then-USDA Secretary Sonny Purdue issued a groundbreaking proposal in December 2020 for a revised regulatory regime appropriately tailored to the research, development and production of GE animals. A month later, the USDA and Health and Human Services, FDA’s parent agency, signed a Memorandum of Understanding specifying that the FDA would defer regulation of genetically altered farm animals to the USDA. The agreement fell apart when the Trump Administration’s FDA Commissioner, Stephen Hahn, refused to sign it in the days before President Biden was sworn in.
President Biden’s USDA reissued the 2020 regulatory reform proposal in March 2021, but nothing was heard about it subsequently until the administration asked in December 2022 for public comments to be submitted by February 2023 on “Identifying Ambiguities, Gaps, and Uncertainties in the Coordinated Framework for the Regulation of Biotechnology.” There has been no word of the status of the proposal since. Our tax dollars (not) at work.
Industry groups, including the National Pork Producers Council, have argued that “for the tremendous potential of gene editing to be safely and readily available to U.S. farmers, regulatory authority for all agricultural applications of these new technologies has to be consolidated at the USDA.”
It’s past time to cut the red tape. Because of the exigencies of both commerce and public health, we need advances like poultry and swine genetically engineered to be resistant to pathogenic viruses. American animal breeders, farmers, and consumers would all be best served by ceding to the USDA exclusive jurisdiction over animals genetically engineered for food.
Kathleen L. Hefferon is an instructor in microbiology at Cornell University. Find her on X @KHefferon
Henry I. Miller, a physician and molecular biologist, is the Glenn Swogger Distinguished Fellow at the American Council on Science and Health. He was the founding director of the FDA’s Office of Biotechnology. Find Henry on X @HenryIMiller