Therapy regimens gradually evolved to treat each of these categories differently, with standard treatments tailored to the specific situation, and this type of increasingly customized therapy has been extremely important. Eventually, we discovered biological differences in cancers that have important implications for patient management.
As our understanding of cancer biology has increased through both basic and clinical research, we now realize that there are many variants within common cancer types (lung, breast, colon, and so on), and medical researchers have been able to exploit those differences to tailor treatment more precisely. Many common cancers are actually a large number of distinct, rare cancer types that all happened to originate in the same organ. Some groups of these are treated in the same way, but for others, very different therapeutic approaches are appropriate.
One tumor of interest is a cancer of the mouth and throat that often arises in non-smoking younger patients. We now know that this type of tumor, which is caused by the human papilloma virus, or HPV, can be cured with much less aggressive therapy than would have been used in the past. It still involves the same modalities — surgery, radiation therapy, and chemotherapy — but at lower intensity, leading to a much better outcome for the patient. Similarly, we now know that cancer of the anus is usually caused by the same virus and can be cured without resorting to surgery and a permanent colostomy.
A useful approach is highly targeted therapies which act on the cancer in different ways, including blocking or turning off signals that cause cancer cells to grow and divide; stimulating senescence in cancer cells – that is, shortening their lifespan; cutting off the blood supply to the tumor; or actually destroying the cancer cells.
Great strides have been made in developing such targeted therapies for certain cancers. For example, about 20% of breast cancers overproduce a protein called human epidermal growth factor receptor 2, or HER2, that promotes the growth of tumor cells. HER-2, therefore, offers an opportune target for drugs, and there are now many targeted therapy options for HER-2-positive tumors.
Similarly, there are drugs that target various genetic types of non-small-cell lung cancer (NSCLC), colorectal cancer, lymphoma, and melanoma. Dozens of targeted therapies are now standard treatments for many of these cancers.
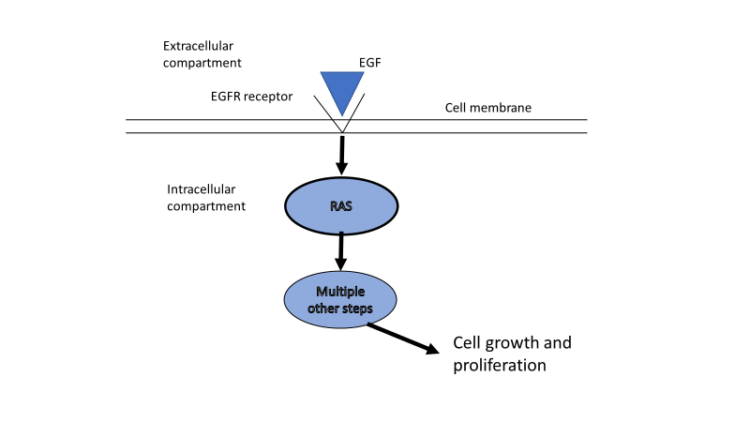
It’s an understatement to say that cancer therapy is monumentally complex. For example, in certain situations we know that a therapy directed towards a specific target will work on some percentage of tumors, but we don’t always know exactly which tumors those are. The disparity can reflect unobvious differences in molecular pathways. A relatively simple example of this is that inhibitors of epidermal growth factor receptor (EFGR) that target HER-1 work very well some, but not all, of the time. It was only after extensive research that investigators discovered that they failed to work when there was an associated RAS mutation (which was quite common), because RAS activates the tumor growth pathway distal to the EGFR inhibition block.
Another important approach is the administration of drugs called immune checkpoint inhibitors, which block a part of the immune system called immune checkpoints and are useful to treat cancers that have certain molecular signatures — specifically mismatch repair (MMR)-deficient cells. These cells have mutations (defects) in certain genes that are involved in correcting mistakes made when DNA is copied in a cell.
MMR deficiency is most common in colorectal cancer, other types of gastrointestinal cancer, and endometrial cancer, but it may also be found in others as well. Because immune checkpoints keep immune responses from being too strong, blocking them stimulates the patient’s natural immunity, enabling it to respond more vigorously to cancer. Knowing that a tumor is MMR-deficient can help in planning treatment and predicting how well the tumor will respond.
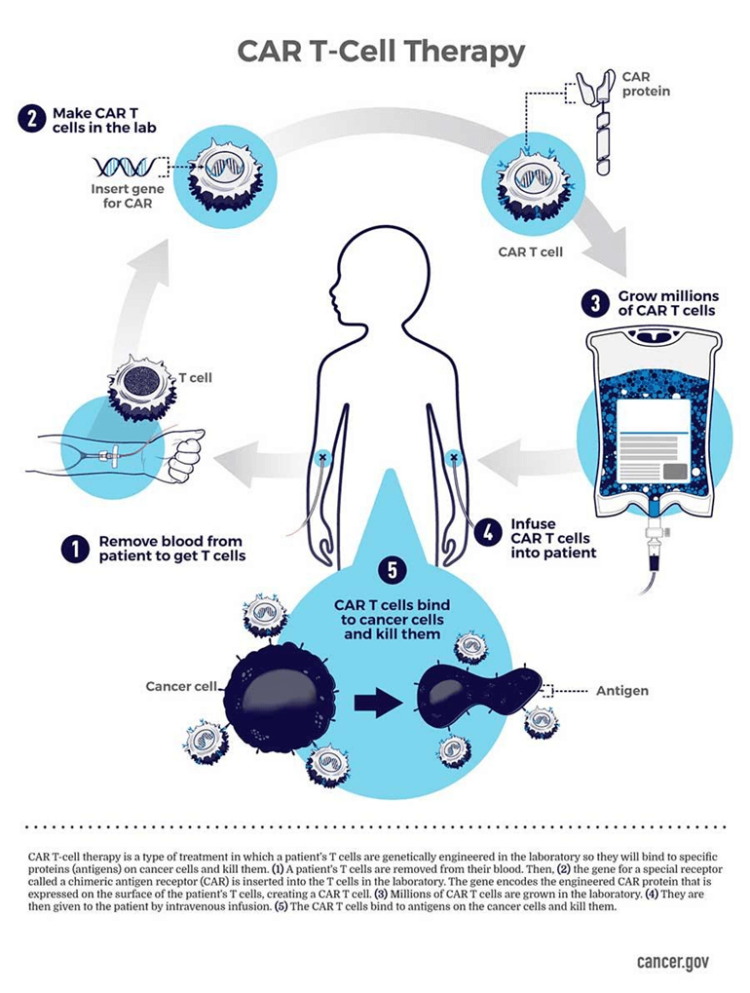
Cancer has become even more treatable with further advances in “personalized,” or “precision,” medicine that have led to treatments unique to individual patients. The prototype, developed during the past decade, is CAR T-cell therapy, an immunotherapy that involves collecting “T cells” – a subset of white blood cells — from the patient and modifying them in the laboratory so that they produce proteins on their surface called chimeric antigen receptors, or CARs, which recognize and bind to specific proteins, or antigens, on the surface of cancer cells.
The altered T cells are amplified into the millions in the laboratory and are then infused back into the patient, where they continue to multiply and, thanks to the CARs, recognize, bind to and kill any cancer cells that harbor the target antigens on their surfaces.
Dr. Michel Sadelain, an immunologist at the Memorial Sloan Kettering Cancer Center in New York and a pioneer of the therapy, describes it as “a living drug — a T cell which has been weaponized against cancer.”
The FDA has approved six CAR T-cell therapies, all of which are used to treat blood cancers, including lymphomas, some forms of leukemia, and, most recently, multiple myeloma. They can be produced currently by only three drug companies.
Over 20,000 people have received one of the six approved CAR-T therapies, and, as reviewed recently in the journal Nature, there are more than 500 clinical trials ongoing underway that are testing engineered T cells to expand the applications of this groundbreaking technology.
Customized CAR T-cell therapy is obviously more complicated and difficult than off-the-shelf drug treatments, and one potential obstacle to its widespread use is the cost, which is in the hundreds of thousands of dollars per patient, excluding the costs of the patient’s hospitalization and related expenses. Academic studies of their cost-effectiveness have been inconclusive, but they are certainly a scientific tour de force.
These new therapies enable more cancer patients to enjoy longer and more normal lives. Progress on all these fronts continues, and as we doctors say after a consultation on a patient, “Will follow with interest.”
Henry I. Miller, a physician and molecular biologist, is the Glenn Swogger Distinguished Fellow at the American Council on Science and Health. He was the founding director of the FDA’s Office of Biotechnology. Find Henry on Twitter @HenryIMiller
Dr. Joel E.Tepper is the Hector MacLean Distinguished Professor of Cancer Research at the University of North Carolina, Chapel Hill. He is the editor of two textbooks, including Clinical Radiation Oncology, and is the founding editor of Seminars in Radiation Oncology.
A version of this article appeared originally at American Council on Science and Health and is posted here with permission. Any reposting should credit the original author and provide links to both the GLP and the original article. Follow ACSH on Twitter @ACSHorg