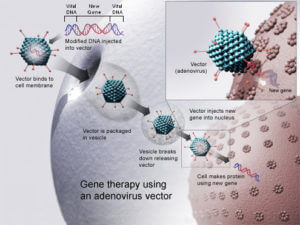
There are new frontiers being explored for genetic disorders of the blood, and they include new approaches in gene therapy that are unique to treating these conditions. In particular gene therapies are being designed that target a patient’s own stem cells in a unique way.
To do this, the patient’s own stem cells are removed, manipulated by inserting corrected forms of the original defective gene, and then transfused back into the patient. This is in stark contrast to the current standard, which is stem cell transplant from a donor (as closely matched as possible, but not identical)–and this involves aggressive chemotherapy conditioning, which carries with it incidences of life-threatening complications, such as graft-vs-host disease. More than three decades of translational science have gone into new gene therapies.
The data, said George Daley, MD, PhD, Director of the Stem Cell Transplantation Program, Boston Children’s Hospital, “show, simply put, that gene therapy works.”
Beta-thalassemia
One of the data readouts presented a recent conference was on gene therapy results treating beta-thalassemia. Beta-thalassemia results in people with reduced production of hemoglobin, which is associated with life-threatening complications, such as severe anemia (due to insufficient oxygen carriers) and organ damage resulting directly from this deficit. Some individuals with beta-thalassemia require frequent blood transfusions but face transfusion-related complications, such as hyperferremia (iron excess), which can itself cause death.
Results were shown from 13 of these so-called ‘transfusion-dependent’ patients who were treated with gene therapy using LentiGlobin BB305 to introduce a fully functioning gene for hemoglobin into the patients’ stem cells. Five of the 13 patients remain transfusion free (meaning that they don’t required this extensive intervention any longer). The other four have had their transfusion needs reduced by more than half but these patients also have two copies of a certain type of HBB gene mutation (beta 0) which suggests that for them to be transfusion-free, they’ll need to have a higher level of corrected hemoglobin. But a reduction by half or more in transfusion requirements is still clinical evidence of the proper effect, and is a huge advance.
Severe Combined Immunodeficiency
Another disease being treated with gene therapy is Severe Combined Immunodeficiency (SCID), also called ‘bubble boy disease.’ Another disease related to genetic mutation (in the IL2RG gene), SCID-Xl is being treated with promising results. Patients with these genetic mutations have poorly functioning immune systems and are prone to life-threatening infections. There were five patients studied who had undergone a previous type of therapy, which is associated with a host of adverse effects. They were then treated with IL2RG gene therapy with lentiviral vectors. The two oldest patients with the longest duration since treatment have increased levels of immune cells with corrected genes. This is exhibiting itself in restoration of immunity in those patients including antibody production, immunoglobulin production, and normal immune responses to vaccines. The other 3 patients have only received the treatment months ago and are being followed-up upon for continued observation.
Wiskott-Aldrich syndrome
Yet another disease treated by gene therapy is called Wiskott-Aldrich syndrome, and is a rare disease caused by mutations in the WAS gene. It only affects males, and WAS gene error stops blood cells from developing properly. Patients with Wiskott-Aldrich syndrome are characterized by low platelet counts, recurrent infections, easy bruising, bleeding, eczema, autoimmune disorders, and higher-than-normal susceptibility to cancer.
Data on 8 children were presented, and all patients were alive after a median follow-up of 3.3 years after treatment (range, 0.1 – 5.4 years). No adverse reactions to gene therapy were observed after infusion. six of the eight patients (75 percent) with a follow-up of more than two years experienced a large reduction in the rate of severe infections and bleeding events after treatment, relative to the rate of such events before gene therapy. These majority of the patients have discontinued anti-infective preventive drug treatments (also not side effect-free) and no longer require a protected environment. Four of the patients (50 percent) no longer receive immunoglobulin supplementation, and of these, two have developed specific antibodies after vaccination. Eczema resolved in four patients (50 percent) and remains mild in two patients (25 percent).
The researcher presenting the data commented that “Importantly, no evidence of abnormal clonal proliferations emerged after gene therapy, and the lentiviral integration profile shows… no skewing for proto-oncogenes.” The importance of this result, showing relative safety from cancerous cell proliferation, is my next caveat below.
New regulation and approach to data collection
Some of the first gene therapeuic studies used adenoviral vectors. These use a modified adenovirus to insert genetic material, but they can cause inflammatory responses and be rejected in the body. This led to retroviral l vectors to be used, but these vectors stopped being used when it was discovered that these retroviral vectors were being inserted into oncogene areas, which caused leukemia in the patients receiving them. This effect wasn’t known when the retroviral I vectors were initially being used, but the data came to indicate that there was about a 25 percent risk of causing leukemia in patients with that technology.
In light of what has been learned in the past, lentiviral vectors are being used now, which not only seem to have a much lower risk (and do not incorporate into oncogenes), but the expression level of the genes is higher, which should theoretically increase the efficacy of all gene therapies using this technology.
These new gene therapies are showing evidence of advancing our treatment of genetic diseases, and while there have been no incidences of cancer to date that have been caused by these therapies, the gene insertion is random, and there is always a risk that the random insertion may end up in an oncogene, and lead to cancerous cell proliferation. This is now why the U.S. Food and Drug Administration (FDA) requires 15 years of follow-up to collect long-term outcomes of gene therapy. There are about five years of longitudinal data so far, so all treated patients will be followed as additional patients are given access to these new treatments. The balance of all the findings, both safety and efficacy, will lead to the conclusive answer of whether gene therapy will represent the continued future of medicine, or if the approach needs to be modified.
Ben Locwin, PhD, MBA, MS, is a contributor to the Genetic Literacy Project and is an author of a wide variety of scientific articles for books and magazines. He is an expert contact for the American Association of Pharmaceutical Scientists (AAPS), a committee member in the American Statistical Association (ASA), and also a consultant for many industries including biological sciences, pharmaceutical, psychological, and academic. Follow him at @BenLocwin.