After a nearly coma-like state of 15 years or so, gene therapy—once the most promising field in the post-genome-sequencing era—may again be showing signs of life. In a field that’s been set back by tragedy, failures, and lots of hype, gene therapy might become a legitimate treatment option.
In the first such treatment to go to the FDA for a new drug application (NDA) nod, a Philadelphia company called Spark has just about completed its phase 3 clinical trials (the last round for testing nearly all potential drugs) on an eye therapy based on an inserted gene. In its trial of 29 people with inherited retinal disease, 93% of them regained sight after receiving therapy that treated the effects of the mutated RPE65 gene. However, the pharmaceutical giant GSK may beat Spark to the gene therapy threshold: it has submitted a gene therapy to the European Union for approval of Strimvelis, a treatment for severe combined immune deficiency (SCID).
Both of these breakthroughs (and that’s what the FDA is calling the Spark therapy, incidentally) come after more than 15 years of developmental doldrums. Ironically, these treatments have arisen from both a disease (SCID) and the site of a tragedy (Philadelphia) that some thought had set back the prospects of gene therapy permanently.
The Philadelphia Story

In 1999, a 15-year-old from Arizona named Jesse Gelsinger, who suffered from ornithine transcarbamylase deficiency (OTCD), a rare X-chromosome-linked metabolic disease of the liver, signed up for a clinical trial at the University of Pennsylvania. James Wilson, the researcher in charge of the trial, had studied OTCD in animals, and in other patients, and was using a form of gene therapy that delivered a “normal” form of the gene behind OTCD, delivered by the same family of virus that caused the common cold; with a few side effects, everybody had so far responded fine in his early clinical trials. Jesse didn’t: shortly after his first injection, he died. The uproar over his death, and further investigations that dug up some mistakes, bad assumptions, and ultimately, an immune reaction the boy had had to the usually benign adenovirus that delivered the gene, shut down gene therapy development at Penn, and nationwide.
In the aftermath, Wilson’s Institute for Gene Therapy was shut down, and he was banned from conducting clinical trials for five years and could not lead a clinical trial for an additional five years. Worse, gene therapy clinical research and development in the United States (but also to some degree in Europe) was stopped. In a 2009 interview with Scientific American, he said, “With what I know now, I wouldn’t have proceeded with the study. We were drawn into the simplicity of the concept. You just put the gene in.”
SCIDing to a stop
SCID, on the other hand, was one of the first diseases to receive the attention of gene therapy researchers. There are several types of SCID, the most common one caused by a mutation in the SCIDX1 gene on the X chromosome. In another kind, children are born without the correct copies of a gene called adenosine deaminase. In these cases, babies very soon start suffering from life-threatening infections and don’t respond to antimicrobials. The first attempts were made in 1990 to insert genes to correct SCID, and other teams had tried to treat various forms of immune deficiency with inserted genes, but the patients developed leukemia, and the trials were dropped.
Genetic changes
What’s changed since then?
-
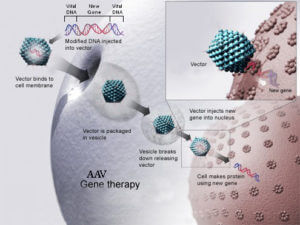
How AAV gene therapy works First, researchers have started working with safer viruses, which don’t trigger an immune response in as many patients. Modified viruses, such as the adeno-associated virus (AAV), can infect just about any cell in existence and trigger an antibody response, but not to the degree seen by adenoviruses used with Jesse Gelsinger’s trial. Also, the retrovirus can also deliver a gene that can be integrated more safely into the genome. And in the case of SCID, researchers are using viral vectors that aren’t as likely to trigger cancer.
- Second, several methods have helped gene therapy delivery overcome natural barriers of cells and tissues. Some of these move beyond gene/viral vectors and include CAR-T therapies, which can use mRNA and genes to more precisely deliver genetic therapy.
- Finally, gene editing techniques, like CRISPR/Cas9 but others like ZFN and TALENS may also provide more precise and varied ways to, if not deliver a new gene, edit or silence the ones already in the body and causing disease. Similarly, identifying genes that can regulate a mutated gene might offer an avenue for switching off that gene, without having to deliver a new, unmutated gene to replace it.
While avoiding the hoopla of its last debut, gene therapy is back. And more promising this time.
Andrew Porterfield is a writer, editor and communications consultant for academic institutions, companies and nonprofits in the life sciences. He is based in Camarillo, California. Follow @AMPorterfield on Twitter.