Four decades after the HIV epidemic began, there’s finally hope it might end. Indeed, “Getting to Zero” — meaning zero new HIV infections — is a slogan used by the World Health Organization and others in fighting the epidemic.
…
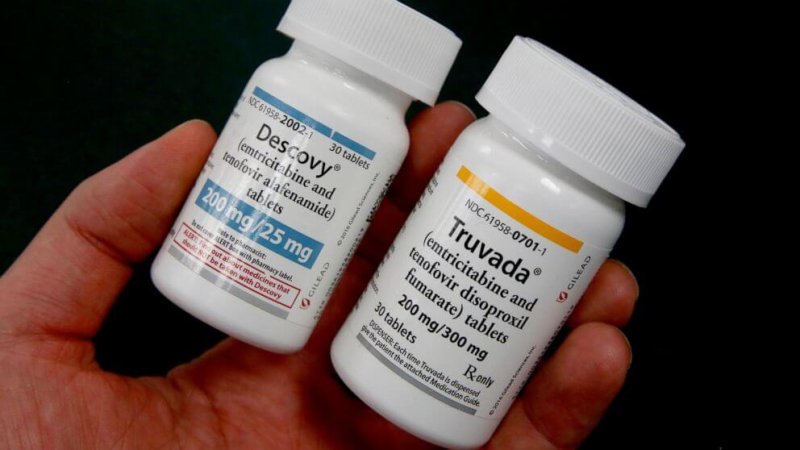
For Gilead Sciences, the manufacturer of the only two FDA-approved pills for [HIV-preventing pre-exposure prophylaxis, or PrEP], the most important zeroes seem to be those the company is adding to its bottom line.
…
Enter Descovy, another PrEP pill from Gilead that the FDA approved in 2019. Generics for Descovy aren’t expected until at least 2022, and possibly 2025. Gilead has been aggressively marketing Descovy for PrEP, including a newly launched ad campaign. We’ve heard research teams — which included Gilead representatives — suggest at scientific conferences that Descovy is not only safer but also possibly more effective than Truvada for PrEP.
…
Hyping Descovy over Truvada for PrEP might lead patients or doctors to hesitate to use Truvada, both the brand-name medication and the generic. That’s not a theoretical concern. Prescriptions for Truvada, approved to treat HIV in 2004, declined substantially in 2016, when Descovy was approved to treat HIV.
There’s no question that having another option for PrEP besides Truvada is a good thing. But when choosing a medication, people should rely on evidence, not advertising.