The first generation of genetically modified (GM) crops has been remarkably successful. The whole world eats food containing ingredients derived from GM crops and feeds them to its myriad agricultural animals and pets. Despite many dire predictions of long-term negative health effects, a quarter century has passed and none have materialized.1 This remarkably clean track record should have assuaged public fears and assured the rapid development and adoption of GM crops of all kinds.
But it hasn’t.
Decades after four major commodity biotech crops – corn, soybeans, cotton and canola – were introduced and rapidly soared to near market saturation in the countries that permitted their cultivation, the number of new GM crops being released to farmers remains tiny.
[Editor’s note: This article is part one of a four-part series on the progress of agricultural biotechnology. Read part two, part three and part four.]
Yet the need for higher yielding, disease-resistant and stress-tolerant crops grows with each passing year. The pressures of population growth and climate warming are already outpacing the speed with which conventional breeding practices are expanding the global food supply.2 Land and water availability are rapidly becoming limiting, hence the focus is sharply on the intensification of agriculture.3 But the breeding methods that fueled the spectacular advances in agricultural productivity over the 20th century are near exhaustion.
Over the same period, knowledge of plant physiology and genetics has grown at an explosive pace, as has the technology for identifying and modifying genes of agronomic interest. We know vastly more about what genes do and how plant genomes change both naturally and under human intervention than we did even when the first GM crops were introduced in 1996.4

The recent invention and rapid development of gene- or genome-editing technology (aka SSN or sequence-specific nuclease technology) has facilitated a quantum leap in the ease and precision of genetic intervention, positioning researchers to accelerate the increase in crop yields and to make crop plants more resilient to the biotic and abiotic stresses exacerbated by climate warming.5
Yet just a few of the crops that need to be improved are being improved using the latest techniques and of those, only a few reach farmers each year. To understand this deep disconnect between what can be done to improve crops using modern molecular techniques – and what is being done – requires a look at the tangle of issues around GM technology at the interface between science, business and society.
In this four-part series, I first examine the factors that led to the disconnect between what can be done and what is being done. I then review both the successes and failures of the first generation of GM crops modified using recombinant DNA (rDNA) technology. I next introduce the new gene-editing technologies and what they promise. And finally, I take a look at the regulatory, political and business decisions that actually determine what gets out of research laboratories and into farmers’ fields. The entire essay is available as a single publication from the author. Please email [email protected].
Part 1: The origins of the disconnect between the science and the farmer
Public resistance to innovation is not unusual, but hardly universal. People line up for the newest Apple iPhone, but have to be persuaded to try a GM apple that doesn’t turn brown. Resistance generally subsides as a technology is widely adopted and proves harmless. GM technology in medicine, for example, is now broadly accepted, be it human insulin or any of the many new protein-based therapeutics. But the controversies around GM crops have persisted, and indeed intensified through the deliberate vilification efforts of both individuals and organizations.6,7

According to polls, the public remains largely ignorant of what GM organisms (GMOs) are and of how modern molecular methods fit into the long history of crop improvement.8 Because fear-based disinformation strategies are so effective, what has grown instead is the widespread conviction that GMOs are “bad,” meaning variously that they are harmful to health, unnatural, or produced by big biotech companies that unfairly exploit farmers.7,9
Part of the problem is that public awareness of genetic modification in agriculture is recent, arguably dating back only to the late 1980s when controversies erupted over field testing of the so-called “ice-minus” bacterium modified to eliminate a protein that promotes ice formation on the leaves of strawberries.10 Yet in a strictly scientific context, “genetic modification” denotes the entire spectrum of human interventions in the genetics of other organisms over more than 10 millenia.11
For crop plants, these encompass domestication, breeding, mutation breeding and, most recently, genetic improvement by molecular techniques. All involve genetic changes, aka mutations. Domestication and conventional plant breeding rely on organisms’ inherent genetic variation.
Direct genetic manipulation of crop plants using chemical and radiation mutagenesis (mutation breeding) dates back to the 1930s.12 But even now, few people other than plant breeders are aware that crops have long been improved through deliberate efforts to induce new mutations using both chemicals and irradiation. So today, it is the general understanding that “genetically modified organisms (GMOs)” are only those that have been modified by molecular methods. That is, most people think genetic modification is quite new.
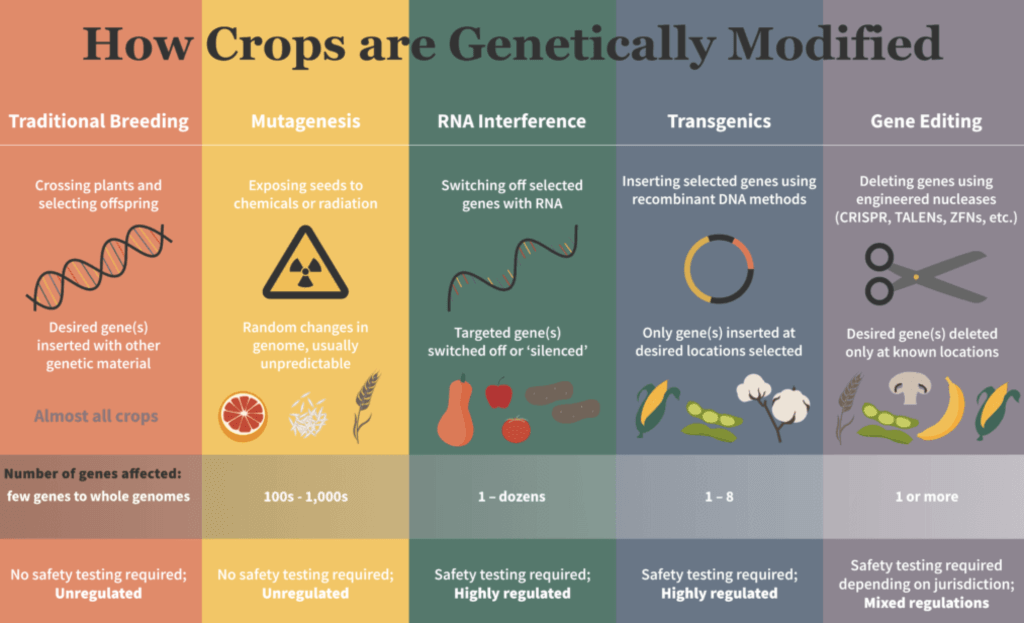
And then there’s what the regulators built
As if this were not sufficiently problematic, the way in which the regulatory environment evolved reinforced suspicions about GM safety. Early efforts to regulate the commercial introduction of GM crops emphasized the need to regulate new crop traits rather than the particular method by which they were introduced. But that’s not what happened.
Starting from the beginning of the regulatory activities in the late 1980s, the U.S. agencies that oversee GM organisms have regulated only organisms modified by molecular methods and they’ve regulated all of them, without regard to either nature of the organism or the trait that was added.13 This has been true of the US Department of Agriculture (USDA) and the Environmental Protection Agency (EPA), although the Food and Drug Administration has generally followed its practice of post-market oversight. None of the agencies subjected new crop varieties produced by the older methods of chemical and radiation mutagenesis to regulatory oversight.
Complying with the regulatory requirements proved not only time consuming and prohibitively expensive to developers,14 but also reinforced the altogether unfounded popular conviction that molecular methodology is dangerous. Both negative popular views of GM foods and the high regulatory costs associated with their introduction have shaped the present availability of GMOs in agriculture. Indeed, it is virtually impossible to understand the contemporary paucity of GM crop varieties without considering both regulatory and acceptance issues.
The recent development of gene-editing methods has led to a new round of public and bureaucratic controversy worldwide over what should be classified as a GMO and subject to regulatory oversight. Because gene-editing techniques15 introduce the same kinds of mutations as the older mutagenesis methods, crops modified by gene editing can be indistinguishable at the molecular level from those improved by mutation breeding.
Mutation breeding has been in safe use for a century, hence there is no scientifically defensible rationale for imposing regulations on crops with the same kinds of genetic changes produced by the new, far more precise methods. This is being recognized in some countries by decreasing the regulatory burden on certain types of crop modifications produced by gene-editing techniques.
However, in 2018 the European Court of Justice ruled that gene-edited crops should undergo the same level of regulatory scrutiny as crops modified by older molecular methods.16 As they have over the past 4 decades, the outcome of such regulatory decisions will profoundly influence the kinds of genetic improvements that will be undertaken and actually become available to farmers and consumers.
Thus both public opinion and regulatory practices have made major contributions to the disconnect between the modern science of crop improvement and the farmer.
1EC (2010). A decade of EU‐funded GMO research (2001–2010). European Commission https://ec.europa.eu/research/biosociety/pdf/a_decade_of_eu-funded_gmo_research.pdf; NASEM (2016). Genetically Engineered Crops: Experiences and Prospects. National Academies of Sciences, Engineering, and Medicine 978-0-309-43735-6 http://www.nap.edu/catalog/23395/genetically-engineered-crops-experiences-and-prospects
2Ray DK et al. (2013). Yield trends are insufficient to double global crop production by 2050. PloS One 8:e66428.
3Tilman D et al. (2011). Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA 108:20260-4.
4Richroch AE (2013). Assessment of GE food safety using ‘-omics’ techniques and long-term animal feeding studies. New Biotechnol 30:351-54; Fedoroff NV (2013). Plant transposons and genome dynamics in evolution. (Wiley-Blackwell, Oxford, UK), p.212; Anderson JE et al. (2016). Genomic variation and DNA repair associated with soybean transgenesis: a comparison to cultivars and mutagenized plants. BMC Biotechnol 16:41.
5Podevin N et al. (2013). Site-directed nucleases: a paradigm shift in predictable, knowledge-based plant breeding. Trends Biotechnol 31:375-83; Zhang D et al. (2016). Targeted gene manipulation in plants using the CRISPR/Cas technology. J Genet Genomics 43:251-62; Zhang Y et al. (2019). The emerging and uncultivated potential of CRISPR technology in plant science. Nature Plants 5:778-94.
6Apel A (2010). The costly benefits of opposing agricultural biotechnology. New Biotechnol 27:635-40.
7Ryan CD et al. (2019). Monetizing disinformation in the attention economy: The case of genetically modified organisms (GMOs). European Management J 38:7-18.
8Funk C et al. (2015). Public and scientists’ views on science and society. Pew Research Center http://www.pewinternet.org/2015/01/29/public-and-scientists-views-on-science-and-society/
9Funk C and Kennedy B (2016). Public opinion about genetically modified foods and trust in scientists connected with these foods. Pew Research Center http://www.pewinternet.org/2016/12/01/public-opinion-about-genetically-modified-foods-and-trust-in-scientists-connected-with-these-foods/
10Palca J (1986). Ice-minus bacteria: Further snag and further delay. Nature 320:2.
11Fedoroff NV (2015). Food in a future of 10 billion. Agricult Food Security 4:11.
12Ahloowalia B et al. (2004). Global impact of mutation-derived varieties. Euphytica 135:187-204.
13Fedoroff NV (2013). Will common sense prevail? Trends Genet 29:188-9; Wolt JD et al. (2016). The regulatory status of genome‐edited crops. Plant Biotechnol J 14:510-8; Van Eenennaam A and Fedoroff N. How the federal government can get biotech regulation right. Des Moines Register, 1 March 2018
14McDougall P (2011). The cost and time involved in the discovery, development and authorisation of a new plant biotechnology derived trait. Crop Life International https://croplife.org/plant-biotechnology/regulatory-2/cost-of-bringing-a-biotech-crop-to-market/
15Kleter GA et al. (2019). Gene-edited crops: towards a harmonized safety assessment. Trends Biotechnol 37:443-7.
16Kupferschmidt K (2018). EU verdict on CRISPR crops dismays scientists. Science 361:435.
Nina V. Fedoroff is an Emeritus Evan Pugh Professor at Penn State University