The discovery of a gene behind the absence of eyes in Mexican cavefish may suggest a new way to treat a rare but debilitating disease in humans– homocystinuria.
In homocystinuria, deficiency of an enzyme (cystathionine beta-synthase a, or CBS), blocks the breakdown of two protein building blocks, the amino acids methionine and serine, while a third, cysteine, diminishes. An array of signs and symptoms result.
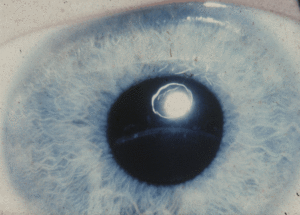
Young children may have slow weight gain and growth, learning disabilities, scoliosis, developmental delay, and behavior and emotional problems. Later symptoms include osteoporosis, chest deformities (a protrusion or caved-in breastbone), and severe nearsightedness and dislocation of the lenses, which can lead to blindness.
Some symptoms of homocystinuria are more serious, including blood clots (increasing the risk for stroke, heart attack, pulmonary embolism, and blocked veins), seizures, hemorrhage, and aneurysm (burst arteries). Premature death is a possibility.
People with homocystinuria tend to be tall and thin with pale hair and skin and long, skinny arms, legs, and fingers. It is a single-gene condition inherited from parents who are carriers. The gene encoding cystathionine ß-synthase a is cbsa.
Mutations in four other genes can also cause the condition, but the subtype due to abnormal CBS is the most common. Still, homocystinuria due to CBS deficiency is rare, affecting from 1 in 200,000 to 1 in 300,000 people in the US. It’s more common in Norway, where 1 in about 6400 people has it, and in Qatar, where it affects about 1 in 1800 people.
A rare fish
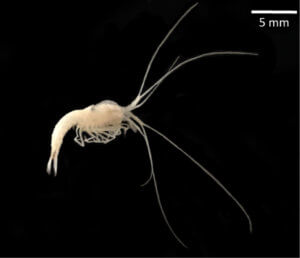
Mexican cavefish have long been a mystery. Some populations live in surface waters and have eyes, like any other bony fish. Yet at least 30 other populations live in cave waters, and the eyes of those fish mysteriously stop developing when embryos become larvae.
In the fish, members of Astyanax mexicanus, paired retinas and lenses begin to form but the cells die and the fledgling structures recede, becoming covered with skin and scar tissue. The tiny shrunken eyes are vestigial organs, like the hindlimb buds of pythons and wing nubs in flightless birds and insects.
Other animals that dwell in caves have shed their eyes over evolutionary time. The no- or tiny-eyed include moles, spiders, beetles, lobsters, crabs, lizards, crickets, worms, shrimp, crayfish, snakes, and other fish. The subterranean naked mole rats lose their eyes after birth. Species that have lost the sense of sight are called troglobites.
When one sense is impaired or absent, others tend to compensate, and that’s true for troglobites. These animals of the darkness also share lower metabolic requirements, and that’s the key to why the seemingly negative trait of sightlessness persists.
Without eyes, the fish shave off about 15% of their energy use compared to their sighted cousins swimming in surface waters. Natural selection favors characteristics that benefit an organism. Fish with lower energy requirements are more likely to survive to reproduce, thereby passing on the trait.
But the blind cavefish don’t lack eyes simply because they don’t “need” them and somehow willed them away. The trait “may confer an evolutionary benefit for cavefish by eliminating the high energetic cost of maintaining eyes, as vision is useless in the dark cave environment,” the researchers wrote.
It’s the ability to lower energy needs, and exactly how the blindness unfolds in the fish, that may have implications for homocystinuria in people.
Zeroing in on one gene
Pachón cavefish, named for the caves that are their homes in Mexico, are the best genetically studied blind fish, but picking out what’s important among the just-under-a-billion DNA bases of the genome is a challenge.
“We know that genes controlling eye degeneration are scattered all over the Mexican cavefish genome,” said William Jeffery, a professor of biology at the University of Maryland in College Park, co-author of a study published in Nature Communications. “There may be 10 to 20 different genes involved, and this is the first time we’ve been able to pin down one specific gene and show the mechanism at work.”

The team, including collaborators from the National Institutes of Health and Stanford University, identified four genes whose expression changes – producing more or less of the protein – during the time in development when the eyes begin to fade away in the fish destined for blindness. Only one was mutant in all of the populations of blind cavefish but not in the sighted populations on the surface. The gene encodes the aforementioned enzyme, CBS.
The researchers used a classic genetic technique: obliterate the function of a gene and observe what goes wrong. They used the gene-editing tool CRISPR-Cas9 and another knockout technology to remove normal copies of the gene in eggs from sighted fish that live in surface waters.
Sure enough, the eggs with the gene knocked out developed into adults that had tiny eyes or none at all. Other experiments added working copies of the gene into cavefish embryos, and they went on to develop eyes.
Focusing in on the function of the implicated gene, and how the symptoms unfold, is what may provide information important to the human disease that results from the same genetic glitch.
Blocked blood flow
The shriveled visual vestiges of the Mexican cavefish arise from blocked blood flow to primordial eyes in embryos and larvae. Without blood, the delicate organs are deprived of nutrients, growth factors, and oxygen and therefore energy. Development halts.
Like any clogged pipe, though, the blockage leads to a range of problems stemming from the compromised circulatory system: leaky blood vessels, hemorrhages in the eyes, and aneurysms (ballooning and bursting arteries). But the problems are confined to the visual system – the adult fish are otherwise healthy.
It’s good to know, from the cavefish, that the primary defect appears to be in blood flow, rather than an inherent weakness in an artery that might make it burst.
“Mexican cavefish are not only surviving with homocystinuria, they’re thriving,” Jeffery said. “One thing we might be able to understand is how these fish recover from hemorrhages in the eye, which could provide insight into treatments for the disease in humans,” he added.
A hint to the hardiness of the adult blind fish may lie in the fact that the species’ genome includes a second gene that encodes the enzyme, which maintains blood flow outside the eye.
Gene duplication is a well-known evolutionary phenomenon that arises from a misalignment of chromosome pairs during cell division, so that one resulting chromosome gets two copies of the gene and the other has none. Having two copies enables one to “test out” a mutation because the other copy maintains health. Sometimes the second copy mutates itself into inactivity, and is then called a pseudogene. This may be what happened to cbsa, the gene encoding CBS.
The human genome also has two copies of parts of the cbsa gene, neighbors on chromosome 21. Would it be possible to boost activity of the second copy in people? That’s the basis of a novel treatment for another genetic disease, spinal muscular atrophy. The strategy of deploying a second gene is a little like opening a second browser.
A new treatment for homocystinuria could be implemented early because the inborn error is part of standard newborn screening.
A blood spot detects buildup of homocysteine from methionine. Normally the enzyme converts the toxic homocysteine to cystathionine, which in turn is broken down into the amino acid cysteine. The buildup of homocysteine damages blood vessels and the brain. The pathway requires vitamin B6.
About half of patients respond to prescription-strength B6 supplements, which must be taken for life. If that doesn’t help, a diet very low in protein may dampen symptoms – otherwise, the risk of a blood clot is 25% before age 16 years and 50% by age 30 years. Supplements that may help are betaine (which flushes some of the built-up homocysteine from the blood), folic acid, vitamin B12, and the amino acid L-cysteine.
The bigger picture to the cavefish/homocystinuria story is a reminder that the common phrase “humans and animals” isn’t correct – we’re animals too! And we can learn from the oddities of other species.
Ricki Lewis has a PhD in genetics and is a genetics counselor, science writer and author of Human Genetics: The Basics. Follow her at her website or Twitter @rickilewis.































