The new vaccine involves clever, cutting-edge technology. It uses a so-called ‘viral vector,’ which is a harmless virus that has been genetically modified (GM) to include coronavirus genes. When this GM virus is injected into human cells, they make coronavirus proteins that stimulate the immune system to fight any future coronavirus infections.
That follows on the heels of the Moderna and Pfizer-BioNTech collaboration vaccines, which also use cutting-edge biotechnology advances, called mRNA vaccines.
Contradiction and hypocrisy?
“These are fantastic results,” UK Prime Minister Boris Johnson enthused, as politicians across the UK and the European Union queued up to praise the latest breakthroughs and to re-assure citizens of the robust, science-based regulatory approval systems that are in place to ensure their safety as the vaccines are fast-tracked through the approval process. This is the right thing to do.
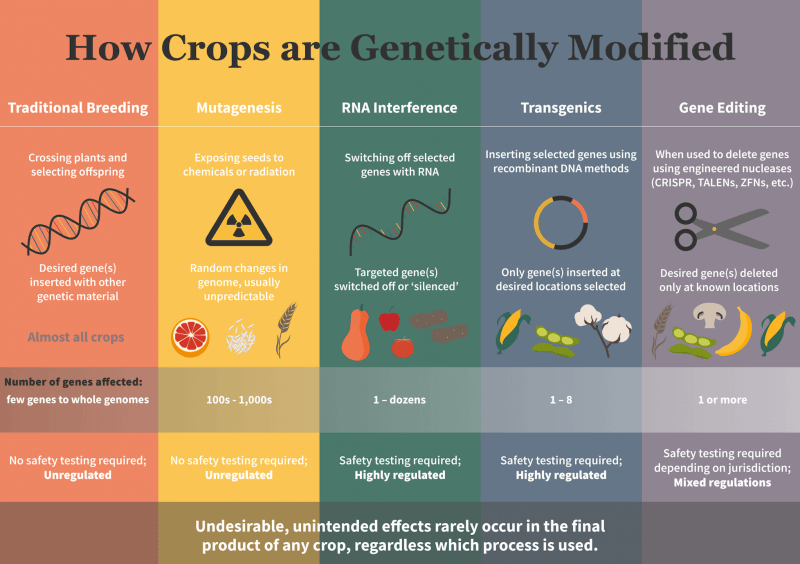
However, wait, isn’t this stance inconsistent and even hypocritical? These vaccines, and a majority of those in the pipeline, are being developed using the very same techniques of genetic modification (GM) or gene editing (GE) that many European politicians have spent the last 25 years preventing their citizens and farmers from having access to for the production and consumption of food, feed and fibre crops and which so-called environmental advocacy groups have opposed unequivocally.
If these politicians and advocacy groups ideologically opposed to GM technology were being consistent with their past 25 years of behaviour, they would be decrying the development and use of these vaccines, campaigning against their approval and publicly stating that they personally will not be using COVID-19 vaccines when they become widely available to citizens.
GMO safety history
Robust regulatory frameworks for undertaking science-based risk assessments of genetically modified organisms (GMOs) have been in place since the 1990s and over 4,300 such science-based regulatory assessments have been conducted in 70 countries by 2019 (ISAAA,2019). These have facilitated the widespread adoption of crops containing these innovations, largely outside Europe, on more than 190 million hectares, in 26 countries by 2018.
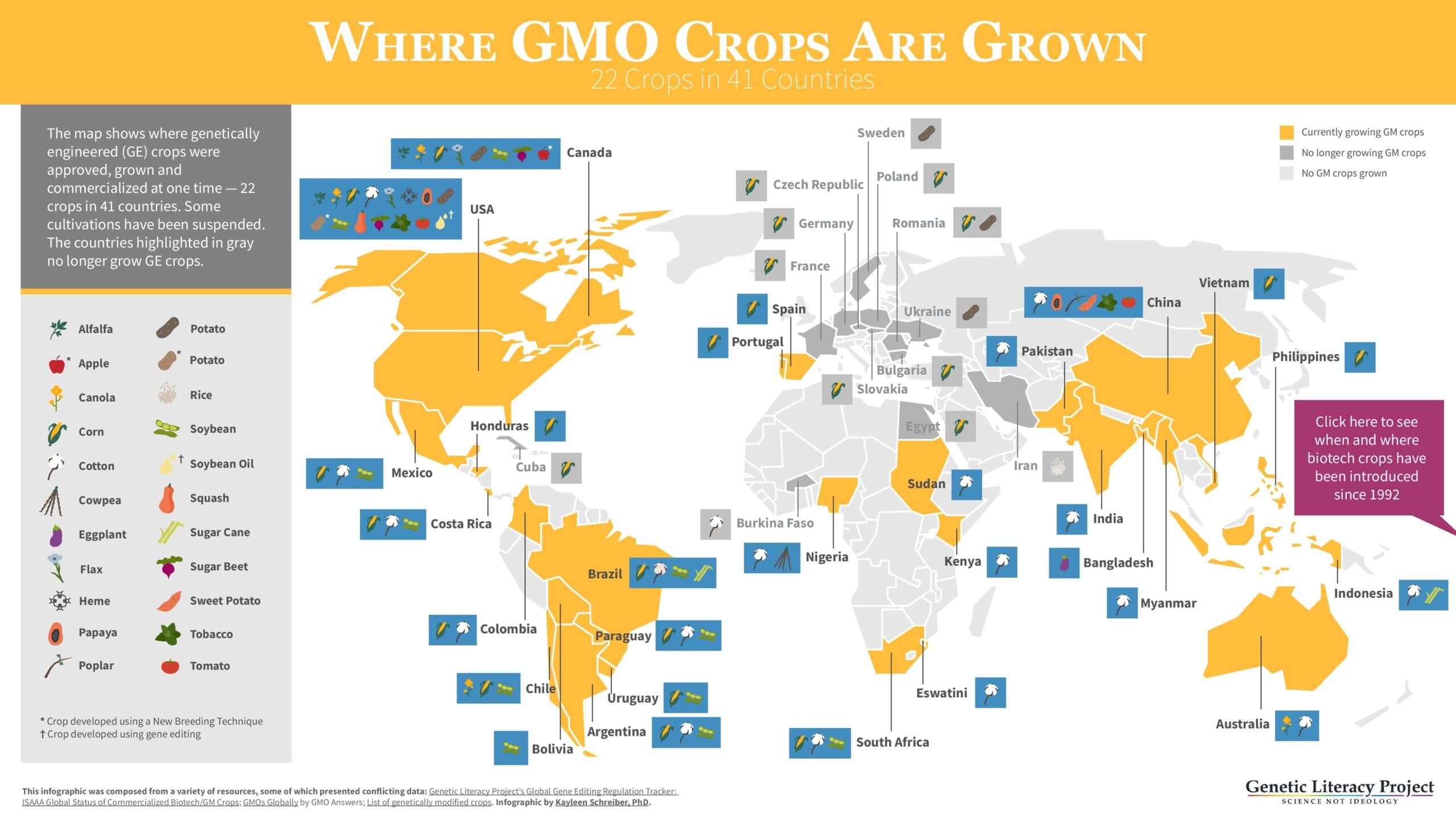
To date, no GM crop or product derived from it has been found to have a level of risk that is significantly different to a conventional crop or product, there has been no credible evidence of negative impact on human health and there is a broad consensus amongst the vast majority of scientists and regulators that these products are safe to consume. More than 280 science-based agencies around the world have endorsed the safety of GM crops.
In addition, there is now a substantial body of evidence, much of which is in reputable peer review publications, that the crop technology has made important contributions to improving global food security, to reducing the environmental footprint of agriculture and to helping to cut global greenhouse gas emissions (e.g., Brookes and Barfoot, 2020).
Despite all this science, risk assessments, monitoring and evidence gathering, most politicians in Europe have continued to apply a non-science and non-evidence based approach to regulating the use of these technologies, largely denying European farmers and citizens access to the benefits referred to above. As a result, the current status of GM/GE crop regulation and use in Europe is as follows:
- In 2020, only one GM crop trait was planted in the EU, an insect resistant maize grown only in Spain and Portugal, essentially a legacy of the early days of GM crop approvals;
- EU Directive 2015/412 allows member states to restrict or ban the cultivation in their territories for non-scientific reasons. Eighteen-member states, and four regions located in two countries (Wallonia in Belgium and Northern Ireland, Scotland and Wales in the UK) have used this opt out to ban the cultivation of GM crops;
- Approvals for the importation and use of GM crops and their derivatives are often subject to uncertainty and long delays due to political interference, often causing disruption to European supply chains of raw materials to European farmers and food/feed processors and users. The average completion time for a GM crop regulatory decision in the EU is five years (Smart et al, 2016) and a group of EU Member States (Austria, Bulgaria, Cyprus, France, Greece, Hungary, Latvia, Lithuania, Malta, Poland, Slovakia and Slovenia) have a long track record of voting against approvals for importation and use, with others, notably Germany, Italy and Portugal usually abstaining from voting. All of these Member States import and use these same GM-derived commodities that their politicians have repeatedly voted to deny them access to;
- The entire European GMO regulatory approval system has been acknowledged as failing to operate as intended (European Commission, 2015) and has been ruled to be mal-administered (European Ombudsman, 2016);
- Lastly, following a Court of Justice of the European Union (CJEU) 2018 ruling, any innovation containing or derived from the use of the latest genomic research technologies known as gene editing (GE) techniques are ‘to be considered the same as a GMO’ and hence subject to the same dysfunctional and mal-administered regulatory approval system. This contrasts with a number of other countries including the US, Brazil, Argentina and Japan which have decided that some (not all, as in the EU) products developed use GE techniques do not merit being subject to GMO-style regulatory approval rules.
From a regulatory perspective, the advent of the coronavirus pandemic and the urgency to develop vaccines has shone a light on the substantial dilemma facing politicians and regulators in European countries concerning regulatory consistency. If there is genuine commitment to deliver consistency in the way scientific developments in medicine and agriculture are delivered, what might the future look like? There are two main choices:
- Apply the same rigorous and solely science-based regulatory approval approach currently being applied to covid-19 vaccines to the regulation of crop and livestock innovations derived from the same technologies so Europeans can have access to the benefits already being utilized in many countries outside Europe in agriculture and food production systems;
- On the other hand, apply the current non-science and non-evidence based regulatory approval approach currently applied to crop innovations that use GM or GE techniques to COVID-19 vaccines. If this ‘overly precautionary approach’ is instituted, the ‘best case scenario’ for Europe is its citizens might get access to the vaccines in about five years’ time, or possibly not at all. This would be an interesting outcome for European politicians to explain to their citizens.
Will the future look like the past in agriculture?
Of course, European countries could carry on with their inconsistent and hypocritical approach of applying a science and evidence-based approach to COVID-19 vaccines and a non-science and non-evidence based approach to crop genetics.
Let’s hope that our politicians take the opportunity that the tremendous development of GM/GE derived vaccines have offered to re-set, re-boot and revise the regulatory approval systems for these technologies so that we can all benefit from their potential in all sectors, including agriculture and food production.
As associate professor in agri-food Innovation at the University of Saskatchewan Stuart Smyth (2020) so aptly recently concluded:
The EU has a unique and rare opportunity to revise its regulatory system in such a way that frees itself from the shackles posed by environmental and political opposition. Adopting a science-based product regulatory system for gene editing technologies that integrates approval decisions with the European Food Safety Authority (EFSA) would reverse the 20-year trend of declining agricultural research and development investment within the EU.
References
Brookes G and Barfoot P (2020) GM crop technology use 1996-2018: farm income and production impacts, GM Crops & Food, 11:4, 242-261, DOI:10.1080/21645698.2020.1779574
Brookes G and Barfoot P (2020) Environmental impacts of genetically modified (GM) crop use 1996–2018: impacts on pesticide use and carbon emissions, GM Crops & Food, 11:4, 215-241, DOI: DOI:10.1080/21645698.2020.1773198
Court of Justice of the European Union (CJEU) 2018. Ruling on case C-528/16 of 25 July 2018. https://curia.europa.eu/jcms/upload/docs/application/pdf/2018-07/cp180111en.pdf
European Commission (2015). Reviewing the decision-making process for GMOs’, Com 2015, 176 final. https://ec.europa.eu/transparency/regdoc/rep/1/2015/EN/1-2015-176-EN-F1-1.PDF
European Ombudsman (2016). Case 1582/2014/php. https://www.ombudsman.europa.eu/en/decision/en/63025
ISAAA (2019) Biotech crops continue to help meet the challenges of increased population and climate change. ISAAA Brief 54. http://isaaa.or/resources/publications/briefs/54/executivesummary/default.asp
Smart R, Blum M, Wesseler J (2016) Trends in approval times for genetically engineered crops in the United States and the European Union. Journal of Agric. Econ. http://doi.org/10.111/1477-9552.12171
Smyth S (2020) Regulatory barriers to improving global food security. Global Food Security. 26, 100440. http://doi.org/10.1016/j.gfs.2020.100440
Graham Brookes is an agricultural economist with PG Economics, UK. He has more than 30 years of experience of analysing the impact of technology use in agriculture and has authored many papers in peer reviewed journals on the impact of GM crop technology. Find him at www.pgeconomics.co.uk































