Motherhood is rewarding, but pregnancy is risky. Pregnant women usually steer clear of environmental risks that can harm the child growing inside them. They avoid smoking, drinking alcohol, or eating fish with high levels of mercury to minimize the risk that their child will be born with a disability. But until recently, women had little power to minimize the likelihood of genetic disorders among their children. The best they could do was select a mate or sperm donor and hope for the best.
For the last few decades, women who used in vitro fertilization (IVF) could scan an embryo for some simple genetic variants that cause diseases like Tay Sachs or Down Syndrome before deciding which embryo to implant. Those are often called Mendelian disorders as they are linked to single genes that caused or increased the likelihood of a disease. But most geneticists have long felt hamstrung because so many diseases are the result of more complicated genetic variations.
Finally, after years of research, with the advancement in whole genome analysis, more subtle mutation patterns can be detected—and science is now poised to transform how we have babies. We are about to enter what might be called the polygenic risk scores (PRS) revolution.
IVF and PGT
Since the 1970s in vitro fertilization (IVF) has allowed fertility specialists to combine a sperm and egg outside of the womb before implanting it. IVF was initially a way for infertile parents to conceive, and later gay couples embraced the procedure. But for the last couple of decades IVF has been combined with pre-implantation genetic testing (PGT) to screen embryos for genetic disorders before deciding to implant them.
Today, more than 20% of embryos used in IVF are tested for simple genetic variants that predispose women to developing cancer and other diseases. An example is the CHEK2 gene, which makes a woman three times more likely to develop breast cancer when compared to women who lack this gene.
Some of the most devastating diseases, like Tay Sachs, arise from a small number of mutations to a single gene. There are an estimated 7,300 such conditions with about half actually identified. They have historically been passed along within populations that remained insular, such as Icelanders, the Amish, Basques or Ashkenazi Jews who are mostly of European descent and intermarried for centuries. Sub-Saharan Africans are more likely to get sickle cell disease; Irish people are more prone to cystic fibrosis, celiac disease and galactosemia; and Ashkenazi Jews are more likely to have babies with Tay Sachs. Children with the most common form of Tay Sachs rarely live past the age of four, and their short lives are made worse by steadily worsening symptoms like blindness, seizures, and choking.

Although these diseases are well known, for most diseases there is not one specific genetic culprit. Despite the huge number of Mendelian diseases, they are relatively rare. That’s because, unlike sickle cell or Tay Sachs, most diseases are polygenic: they result from many genes interacting in ways that make it more or less likely that we’ll get a condition like heart disease or cancer or dementia. And many people develop these diseases only later in life, since polygenic conditions generally result from a combination of genetic predispositions and environmental stimuli like diet, exercise, stress, and even random developmental forces that aren’t yet well understood.
PRS breakthrough?
Despite the complexity of polygenic disorders, a newer technique called polygenic risk scores (PRS) allows women who use IVF to screen embryos for polygenic disorders—and it’s poised to revolutionize baby making. PRS uses data from the entire genome to assess disease risks, rather than scanning embryos for single gene disorders. This makes it more expensive, but it also allows couples an even more powerful way of assessing genetic risks, including a predisposition to heart disease, diabetes, and schizophrenia. While PRS is not new, the massive datasets that allow it to be used more effectively have only emerged over the last few years.
In 2020, Genomic Prediction became the first company to help a couple have a healthy child screened for polygenic diseases. Over the past year Myome and Orchid followed suit, offering similar polygenic screening for couples. Later this year Orchid will offer embryo testing, generating risk probabilities for disorders far beyond the ability of current tests. Other companies will be quick to follow, and many of them will likely offer over-the-counter kits like Orchid does. Couples take the test at home by spitting into a tube and mailing it in. The company sequences the genomes of each parent and matches them against data of people with and without these diseases to calculate their PRS. Later this year Orchid will offer embryo testing, generating risk probabilities for disorders far beyond the ability of current tests, which only evaluate single-gene conditions.
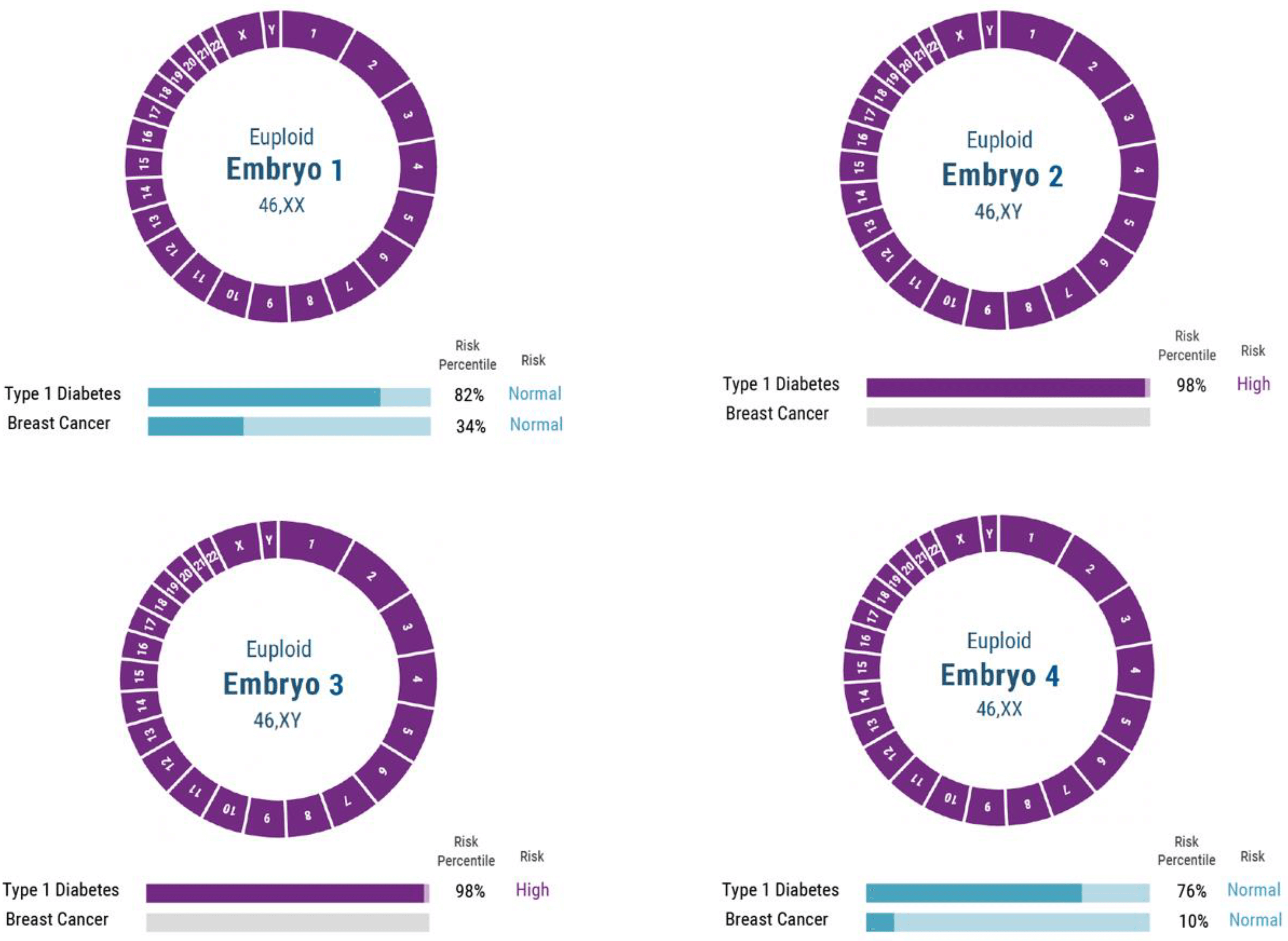
This is clearly a breakthrough development, but is it ready for prime time? There are medical concerns about potential unintended consequences, and moral concerns about misguided attempts to create ‘perfect’ children, as well as the emergence of genetic injustice as a result of unequal access to the new technology. Will this usher in an era in which parents can not only eliminate embryos likely to carry certain diseases but also choose the physical and psychological traits of their kids?
Medical risks and ethical concerns
One objection to using PRS to predict disease risk is that some genes – known as pleiotropic genes – have multiple effects on the how the body works, our phenotype. The worry is that by selecting an embryo with less risk of disease we may inadvertently produce a ripple effect, an unknown harm to the child.
In an editorial last month in The New England Journal of Medicine, twelve physicians and social scientists warned that “as polygenic scores improve and reproductive technology advances… the magnitude of its unintended consequences may also increase.”
Some others have echoed this sentiment. In an opinion piece for Scientific American, Laura Hercher writes:
Polygenic risks scores attempt to sum up the overall likelihood of a particular outcome—such as getting a disease—by simply observing which patterns of variation in a genome are associated with a higher or lower probability of having the condition. In other words, this method gives us information about who might be more or less likely to get sick without explaining why. The statistical association is real but hardly definitive, and it tracks population level trends that may not be relevant for the individual in question.
It is trite but true to observe that causation can’t be inferred from correlation. But it is also true that patterns of correlation are precisely how we make causal inferences in the sciences, even if correlation isn’t sufficient for us to infer causation. More to the point, every new technology has some uncertainty and some unintended consequences, which gives us reasons for caution. But caution shouldn’t paralyze us, and we shouldn’t exaggerate the uncertainties.
There are two reasons the pleiotropy objection may be overblown. First, and most important, selecting against diseases that involve pleiotropic genes can be good rather than bad. There are reasons to believe that some of the same clusters of genes reduce risks for several different diseases rather than reducing risks for some while raising it for others. This means that there may be unintended positive rather than negative effects of selecting against elevated risks for psychiatric diseases like schizophrenia. As Laurent Tellier and his co-authors argue, selection against some diseases often “simultaneously reduces the risk across the entire panel of disease conditions, presumably because some of the same variants are implicated in a variety of conditions that impair physical and mental health.”
Second, every decision we make involves risks, including the decision to get pregnant. The need to minimize unintended consequences associated with embryo selection highlights the importance of genetic counseling, which can help parents interpret the science, so they can better understand what is known and what is not known without imposing undue constraints on choice. More generally, the complexity of embryo selection with PRS illustrates the importance of increasing knowledge about genomics in the general population, especially among parents. As the NEJM authors argue, we should encourage a broader conversation about this new technology.
Devaluing life?
The authors of the NEJM editorial also worry that widespread use of embryo selection using PRS could end up “altering population demographics, exacerbating inequalities in society, and devaluing certain traits.”
We disagree with the authors’ claim that selecting against disability or disease will somehow “devalue” those who already have these traits. We can create a culture that treats people with disabilities with dignity – whether the disability results from genetics or from injuries sustained in an accident – but also recognize that disabilities and diseases are conditions we should try to avoid. We already routinely alter our environments in order to make disease and disability less likely. For example, we pay additional money for safety features in cars, and additional taxes to have guard rails installed on highways in order to reduce car crashes and thus disabilities.
We should also allow prospective parents to select embryos in ways that minimize the risks of people developing diseases or disabilities. We already do this for Down Syndrome, and we see no reason why we should force parents to choose embryos at random simply because some people already exist who have genetic diseases.
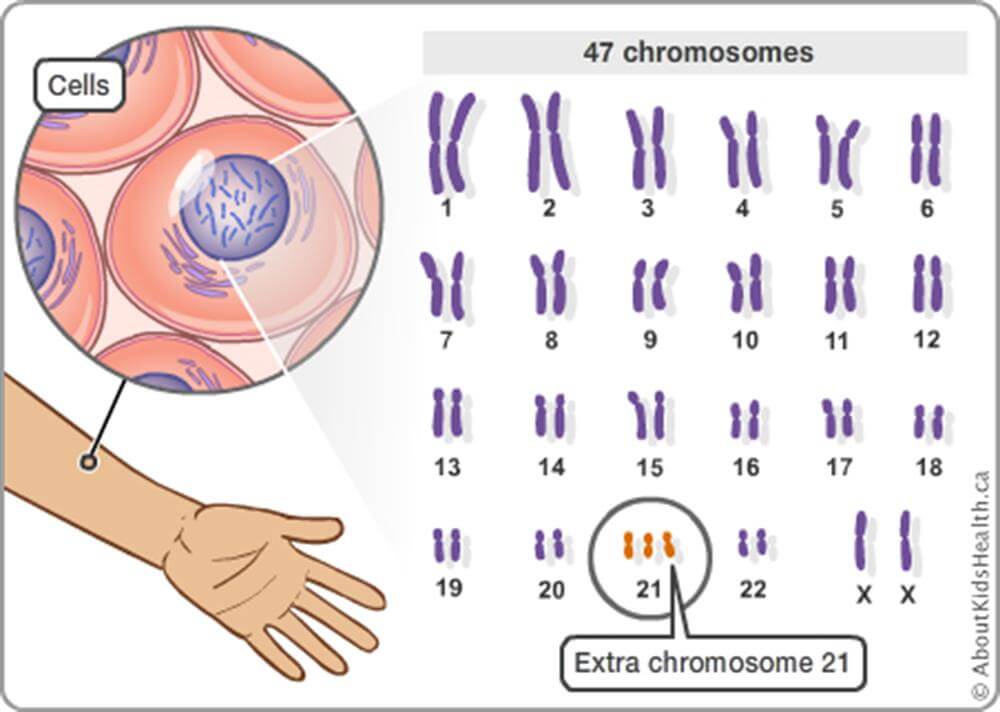
In fact, throughout the Western world laws protecting the disabled have increased, along with attitudes of empathy toward the disabled, at the same time that couples have used IVF and PGT to select against certain disabilities. So, it is empirically false that selecting embryos to minimize disability will automatically increase hostility toward those who have disabilities. Indeed, understanding the genetic basis of some disabilities could make us more compassionate toward people who have them.
Even if some parents would prefer not to test embryos for disease risks or disabilities, we do not believe it gives them the right to use the power of the state to prevent other parents from doing so. Such restrictions would reduce real opportunities for women to have children with the best chance of the best life.
Designer babies and genetic inequalities
What about the concern that PRS could put us on a slippery slope to a Brave New World of designer babies? After all, parents already go to enormous lengths and spend lots of money to give their children advantages. At this point, this is mostly a theoretical concern. Our current technology is a long way from enabling parents to create ‘designer babies’. So far, it just allows them to lower the probability of certain monogenic or polygenic diseases.
There is a danger of miscommunicating what we know and do not know about genetic inheritance. When genetic predispositions are discussed, physicians and patients sometimes discount the role of the environment in the womb and after birth. This is why general scientific literacy is important, and why genetic counselors will be essential.
Given the novelty of PRS, some may still be tempted to advocate a ban or a set of severe restrictions on the technology. However, the more powerful embryo selection becomes, the more likely it will be that restrictive laws against embryo selection will cause black markets for embryo selection to emerge. Eventually, as the science advances and the technology becomes more powerful, some parents will want to augment physical and psychological traits like height and intelligence, not just minimize common disease risks like cancer.
While we think there are benefits and costs to enhancing some of these traits, restrictive laws will backfire, increasing genetic inequalities by creating black markets that only the wealthiest and most genetically privileged parents can afford to access.
There are also ethical reasons to be concerned about over-regulation. Restrictions against embryo testing would be inconsistent with how we treat other aspects of reproductive choice. We already allow all pregnant women access to prenatal genetic testing. Is it really more ethically problematic to allow parents to choose an embryo to implant than it is to allow parents to terminate a pregnancy on the basis of a genetic anomaly?
The years ahead
It is likely that a myriad of genetic screening companies like 23&Me – which already provide parents information about heritable disease risks – will eventually offer parents the tools to test, select, and alter embryos to minimize disease and influence other traits. Insurance companies and governments may eventually subsidize access to genetic counseling to allow parents who want to access such information the ability to interpret it just as they do now for IVF.
As with most areas of life, there are no guarantees. But PRS may help women minimize the chance that their future children will suffer unnecessarily. It is not a magic bullet; embryo screening will not protect kids from the many dangers they will face during and after childbirth. But it gives parents a chance to prevent the kinds of diseases that have devastated family members, and gives children a better chance of surviving and thriving into adulthood.
If we already trust people to make decisions about having children, we should also trust that parents are better placed than bureaucrats to use genetic information to inform their reproductive choices.
[Editor’s note: For a contrary perspective, please read What makes one embryo ‘better’ than another? Why selecting children through polygenic scoring might not work as intended on the GLP]
Jonathan Anomaly teaches classes in game theory and ethics at the University of Pennsylvania. He is the author of Creating Future People: The Ethics of Genetic Enhancement.
Diana Fleischman is an evolutionary psychologist and associate research professor of psychology at the University of New Mexico.