Thus, the two approaches defined as “twins” just a few lines previously are reduced to a single offspring: the transfer between countries of technologies which are useful for biodiversity (recommended by Art. 16 Convention) has disappeared; the Protocol will deal solely with the risks which are hypothetically linked to transboundary movements of “living modified organisms” (Art. 19 Convention): “there has been a significant shift from a positive affirmation of biotechnology’s role in the goals of biodiversity to a complete disregard for its role in meeting the Convention’s objectives.” (Smith 2000, p. 31) Therefore an orientation is confirmed which dates back to prior to the signing of the Convention on biodiversity, i.e. an ultra-regulatory approach, supported by exponents of rich countries also against the interest of poor countries in biotechnology: a “curious dialogue, between developing country scientists asking for access to biotechnology and its benefits; and environmental experts from the developed world insistently offering regulations.” (Cantley 1995, p. 644).
In addition, there is a robust rereading of the Precautionary principle: while it states that precautionary measures cannot be denied if there is an imminent danger for the environment, with “threats of serious or irreversible damage” (United Nations 1992, Principle 15), even if not (yet) fully confirmed scientifically, here the principle becomes an imperative to treat the indicated material with care, without explaining why, as potentially dangerous. The misunderstanding has therefore existed right from the start: what is the basis for the original supposition to which the Protocol refers, i.e. that organisms modified by modern biotechnology (for which read: “GMOs”) are in themselves a possible risk for biodiversity and human health, such as to deserve a separate, wary approach, and which moreover require an international treaty? In what way would they be essentially different from all the less “modern” biotechnological modifications or, for that matter, from other contemporary gene-improving techniques (e.g. tissue culture or polyploidy induction) which do not fall under the legalistic “GMO” umbrella?
Discussion of this allegedly harmful uniqueness is peripheral to or completely absent from meetings of negotiators (Ammann 2014, p. 7); this suspicion of serious risk is therefore taken for granted, and all the daunting legal machinery is based on this presupposition, which is not justified at all: “the core assumption of the protocol – indeed, its raison d’être – is that DNA recombinant techniques in agriculture pose more potential risk than other means of transforming cultivars.” (Herring 2010, p. 84) The perfectly valid Precautionary principle is abused (Tagliabue 2016a): bereft of its underpinnings – scientificity, proportionality and non-discrimination in the approach to the hypothesized risks – it is used explicitly as an “anti-GMO” tool.
Again: the inadequacy of the Protocol lies at its very basis: “mixing ‘process apples’ with ‘product oranges’ has lead to a great deal of fear and confusion. For the precautionary principle to be truly effective, it needs to be applied to all novel organisms, and not just to those developed using modern biotechnology.” (Buechle 2001, p. 305)
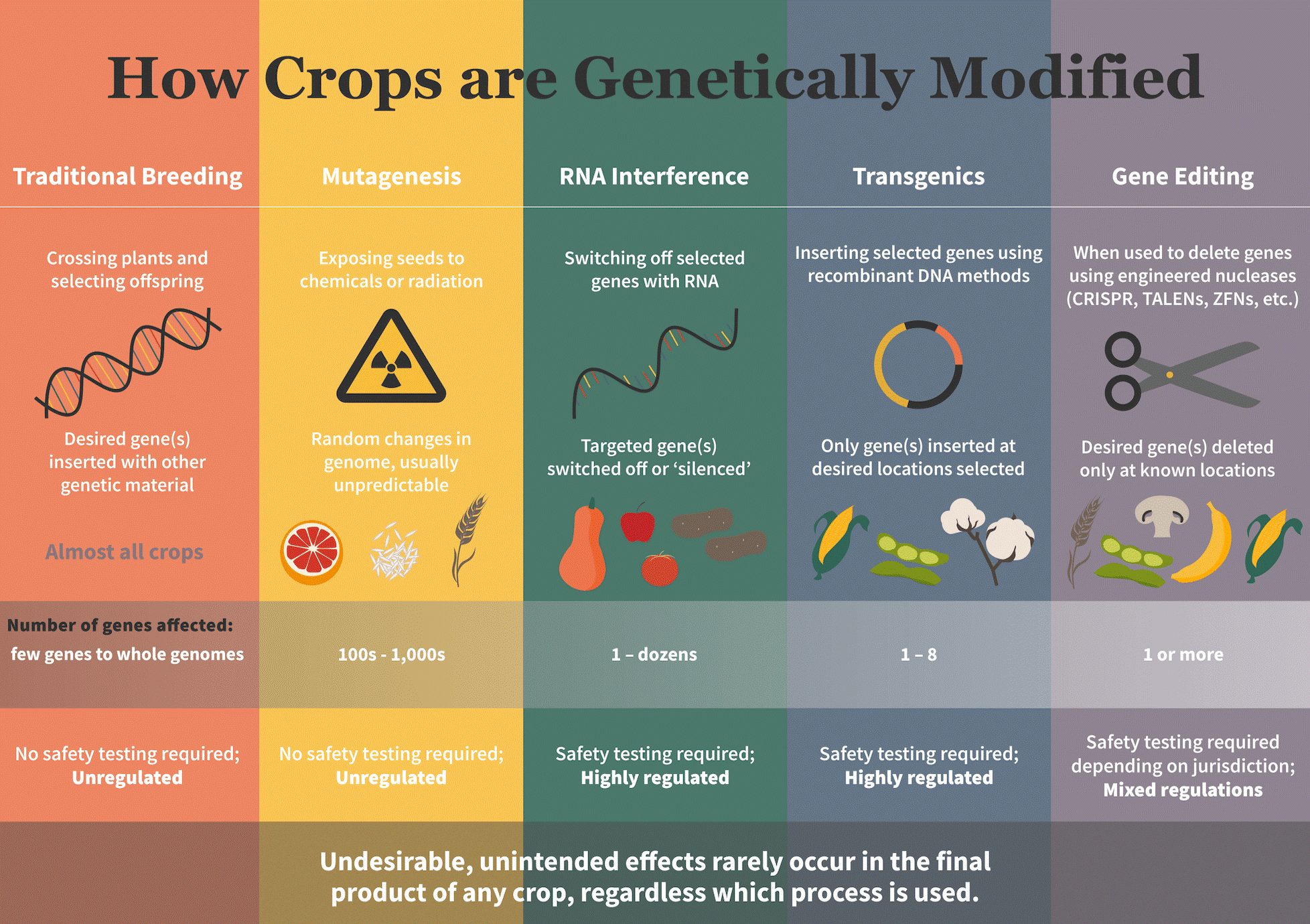
“GMOs” are here renamed “LMOs” (living modified organisms), in other words “any biological entity capable of transferring or replicating genetic material, including sterile organisms” (Art. 3. Use of Terms – h. We will see how this indicates some “GMOs” and not others). But what are these objects, which must be handled with such care and fear? Using an equally pointless and inevitable repetition, they are defined as “any living organism that possesses a novel combination of genetic material obtained through the use of modern biotechnology” (Use of Terms – g). How can these organisms be identified, and what is this “modern biotechnology”? Here we come across a botched definition:
“’Modern biotechnology’ means the application of:
a. In vitro nucleic acid techniques, including recombinant deoxyribonucleic acid (DNA) and direct injection of nucleic acid into cells or organelles, or
b. Fusion of cells beyond the taxonomic family, that overcome natural physiological reproductive or recombination barriers and that are not techniques used in traditional breeding and selection” (Use of Terms – i).
Thousands of cultivars and ornamental plants created via other biotechnological modifications, e.g. chemical or physical mutagenesis (FAO-IAEA 2017), are excluded from the scope of the Protocol. Many products, which are modified to obtain the same trait via non-rDNA kinds of genetic interventions, e.g. tolerance to herbicides (BASF 2018) are not covered by the “modern biotechnology” arbitrary definition. No explanation is given: those who wrote the text of the Cartagena Protocol decided to regulate not the products, rationally assessing the risks and benefit of each cultivar according to its characteristics, but certain processes – which are, in addition, necessarily ill-defined. Indeed, the principle by which the correct criterion is to regulate the product, regardless of the process, was warmly recommended right from the start by the most important scientific bodies; it is sufficient here to note the position of the European Molecular Biology Organization: “EMBO strongly believes that there is no scientific justification for additional, special legislation regulating recombinant DNA research per se.
Any rules or legislation should only apply to the safety of products according to their properties, rather than according to the methods used to generate them.” (1 October 1988, 40th meeting of the Council of the EMBO, cit. in Cantley 1995, p. 560) Also at international level, a basic document issued by the OECD was very explicit: “There is no scientific basis for specific legislation for the implementation of rDNA techniques and applications.” (OECD 1986, p. 42) But not even two subsequent letters (See Cantley 1995, p. 560-561) in 1989 and 1990 to the decision-makers of the then European Community on the part of fully sixteen European Nobel prize winners for medicine or chemistry who were concerned about the future of research and development in the sector of recombinant DNA, managed to convince politicians to abandon the path of special regulation for “GMOs”: such an unscientific approach then took the form of national and European ad hoc regulations, confirmed by the Cartagena Protocol.
In passing, we note that the area of application of the Protocol is limited to products linked to the biotechnologies of “green” recombinant DNA, food related or otherwise: it confirms the welcome but unjustified exclusion of the similar “red” products, i.e. medicines, which are dealt with by other relevant international agreements (Art. 5. Pharmaceuticals).
As for the measures applied to cross-border trade of “LMOs”, each movement must be communicated to a Biosafety Clearing-House, a central database which is shared among all the Parties, i.e. the signatory countries (Art. 20). Preliminarily, it is noted that the Protocol does not apply to material in transit (i.e. in a transit country between the exporter and the importer) or destined to be used in closed environments, unless the individual states, on one issue or another, decide otherwise (Art. 6. Transit and Contained Use): this opens up the way for any national jurisdictions which choose to be imperatively “anti-GMO”. Then there is a host of requirements which are jointly termed the “advance informed agreement procedure” (Art. 7 ff.), which covers the indications for the management of “LMOs” in an open field (“for intentional introduction into the environment of the Party of import”): on the first transboundary movement of a particular “LMO”, the exporters must notify their intention in writing to the competent authority of the importing country; the latter must acknowledge receipt within 90 days; but if it does not do so, this does not imply agreement to the operation (this explicit negation of the reasonable rule of “silence equals consent” puts import-export operators at the mercy of capricious bureaucrats). The decision on whether to give agreement must be communicated to the applicant within 270 (sic) days, but if this deadline passes it can be further delayed; if the import is denied, there is no way to challenge the decision or to turn to independent arbitrators. If agreement is given, it can in any case be withdrawn subsequently, at the discretion of the importing country (Art. 12).
The advance informed agreement procedure explicitly does not include “LMOs intended for direct use as food or feed, or processing (LMOs-FFP)” – without prejudice to the fact of, in any case, informing the Biosafety Clearing-House of the transaction (Art. 11); this establishes a further convoluted sub-distinction between loads of seeds for direct food consumption or use as raw material and those for cultivation (the “introduction into the environment”). Now it is possible – in part – to understand the strange change in terminology from “genetically modified organisms” to “living modified organisms”: the negotiating success of the countries that wanted fewer restrictions on the movements of “GMOs” has led to some marked exceptions to the burdensome procedures imposed by the Protocol, even if this entails another intricacy in terms of definitions and strengthens our considerations on the extreme oddness of the “GMO” bogus category. In fact, recombinant DNA maize grains are living organisms, flour is not; coffee beans from plants which are drought resistant are, ground coffee is not; cotton plants which tolerate herbicides are, the final product is not; papayas made immune to viruses are, papaya juice is not; genetically enhanced edible rape seed is, the oil from it does not even contain DNA. Derivative products, which are not “living”, do not therefore fall within the scope of the Protocol whereas it does include, but in a bureaucratically less burdensome way, “living” organisms which are destined to be transformed, not cultivated.
The text continues with a plethora of often repetitive clauses, in which every so often the distorted interpretation of the Precautionary principle is reaffirmed; the treaty, in reiterating several times that the Parties are in any case free to add further obstacles to the cross-border circulation of “LMOs”, leaves very broad scope for the decision-making intervention of politicians from every nation: “The anti-science treaty empowers governments to ban biotechnology products without any evidence of harm. All they need are expressions of concern. In other words, they can impose a ban simply because they have given themselves the power to do so.” (Juma 2013) In fact, the references which spring up here and there to the scientific solidity which must drive decisions – for example in the risk assessment and management of “LMOs” – seem merely rhetorical; a generic precautionary reference to the lack of evidence of the non-risk nature of the products that a nation wishes to block is enough: “the biosafety protocol establishes an ill-defined global regulatory process that permits overly risk-averse regulators to hide behind the precautionary principle in delaying or deferring approvals.” (Miller and Conko 2000, p. 360)
Moreover, the official website of the Protocol recognizes that it deals with issues which are also covered by international agreements signed by many countries at the level of the World Trade Organization (http://bch.cbd.int/protocol/cpb_faq.shtml#faq27): on this point, the preamble of the treaty states that it does not imply “a change in the rights and obligations of a Party under any existing international agreements”, but immediately it adds that this must not be interpreted with the intent “to subordinate this Protocol to other international agreements”. The opinion of some critics is that the treaty goes on about risks for the environment and health, but in reality “is solely concerned with establishing the rules under which countries can limit imports.” (Hobbs, Hobbs and Kerr 2005, p. 281)
In short, there are fairly open protectionist intentions: and if disputes arise, it is far from clear whether the international law on free trade which countries have signed at WTO level must prevail, or rather the impediments which the countries involved in movements of “LMOs” decide to impose on these peculiar wares. The remarks on this issue may be scathing: Cartagena “makes a travesty of international law. […] the parties to the Biosafety Protocol were unable to agree on the priority or precedence of the Biosafety Protocol over other trade treaties. […] Consequently, the Cartagena Protocol is a corrupted treaty that adopts the impossible and absurd position of both affirming and rejecting earlier obligations.” (Guruswamy 2002, 491-492)
Certainly, the best thing would be to find interpretations which do not contradict each other: “Where two parties in dispute are both members of the WTO treaties and the Cartagena Protocol on Biosafety, attempts should be made so as to interpret the treaties to try to make both applicable.” (Kinderlerer 2008, p. 37) However, it is difficult to have your cake and eat it, as has been learned from the experience of the dispute over the import-export of “GMOs” between the EU on the one side and Argentina, Canada and the USA on the other, which the laborious WTO arbitration resolved in favour of the first two (WTO 2006. Excellent summary of the case: Bernauer and Aerni 2008), while the dispute is still open with the third (WTO 2010). It is certainly no coincidence that the three great states from the Americas are the key absentees from the list of signatories of Cartagena (although Argentina and Canada have ratified the “mother” Convention): those countries avoided putting themselves in the hands of an international treaty which gives signatories the right to establish unjustified obstacles to the import of goods which the three non-contracting countries produce in abundance.
The legal scenario is therefore confused. And it is confused not only in regard to the possible conflict between the WTO and Cartagena (Kerr, Smyth, Phillips and Phillipson 2014), but the glaring contradiction emerges between the same Convention on biodiversity and its first supplementary protocol. In fact, the main treaty says: “Trade policy measures for environmental purposes should not constitute a means of arbitrary or unjustifiable discrimination or a disguised restriction on international trade.” (United Nations 1992, Principle 12) The Cartagena Protocol disregards this basic principle. An excellent “parent” treaty, which however brought with it the risk of degeneration of the “offspring”, has effectively created an abnormal regulation, in which the hoped-for transfer of biotechnologies that can help biodiversity is completely abandoned, while the bureaucratic edifice is built on a detrimental prejudice and, moreover, triggers the potential for opening up conflicts between treaties in the case of commercial disputes.
The Protocol commits the richest contracting parties to providing financial support, in implementation of the procedures, to the economically weakest, “in particular the least developed and small island developing States” (Art. 22. Capacity-Building, Art. 28. Financial Mechanism and Resources). But the poor could make better use of the funds made available to them, instead of investing (wasting!) money on eagle-eyed supervision of import-export mechanisms which herald risks only in the minds of “GMO” opponents: “The resources spent on negotiating the protocol and how to implement it would be better spent on more pressing global problems, such as boosting the developing world’s capacity to use science for development.” (Ventura 2006) (2) The insistence on the concept of capacity building is clearly wrong: teaching low-income countries to implement effective safety standards to limit damage which is completely hypothetical and, as “LMO-GMO” crops and products are increasingly produced and consumed around the globe (ISAAA 2019), manifestly unfounded, makes no sense. Cartagena presents itself to the World Health Organization as one of the burdens which (pointlessly) provide a tough test for the already insufficient bureaucratic structures of poor countries: “At the international level, 15 legally binding instruments and non-binding codes of practice address some aspect of GMO regulation or trade. Such sector-based regulations increase the already overstretched capacity of developing countries.” (World Health Organization 2005, p. iv)
The financial resources, which are not infinite, would be much better used to teach Third World farmers how to handle phyto-pharmacological products, i.e. herbicides and pesticides, which are often deployed without adequate safety and protection measures for users: instead of using their scarce funds to equip themselves against imaginary dangers, the resources should be used to face and handle risks which are very clear and well established. Instead, “the Global Environment Facility (GEF), as the funding mechanism for the Convention on Biological Diversity, has provided considerable funding to implement the Biosafety Protocol” (International Union for the Conservation of Nature 2007, p. 40). Thus, a significant amount of public money is not directed to financing the defence of biodiversity which is truly threatened, but to introducing and supporting redundant bureaucracy in order to implement complicated procedures deriving from a prejudicial mega-treaty.
Such radically negative opinions on the Protocol were expressed by expert critics at the time of its approval: “The sad irony of the Biosafety Protocol is that it may well retard, rather than advance, the protection of biodiversity. Under the guise of adopting ‘precautionary’ measures to protect the environment, the Protocol could restrict one of the most important tools for biodiversity conservation – agricultural biotechnology.” (Adler 2000b, p. 5) The obstacles posed to movements of the most productive crops, i.e. those “GMOs” which enable higher yields on a given cultivated surface area, will inevitably lead to future agricultural pressure on land which is still unused, and consequently the destruction of habitats will impose further damage on biodiversity: “Unless agricultural productivity increases substantially, this will mean putting thousands, if not millions, of additional hectares under plough – and consequently losing thousands, if not millions, of hectares of species habitat. Thus, a failure to enhance per-acre agricultural productivity will have severe consequences for global and regional biological diversity.” (Adler 2000b, p. 10) This is the combined effect of the constant population increase and the rejection of biotechnologies which, to a greater or lesser extent, could improve and increase agricultural production, and thus reduce the use of previously untouched land. While we remember that the greatest damage to biological diversity is the lost of habitats, we note that a regulation, which claims to derive from the Convention on biodiversity, means precisely the negation of it.
It is not surprising then that the Executive Secretary of the Convention, the leading African scientist Calestous Juma, resigned from his position before the Protocol was approved; he could not stand its clash with the “mother” treaty and the embarrassment in terms of logic as much as law: “he didn’t want to see the Cartagena Protocol on Biosafety passed under his watch”. (Allen 2013)

Is there any way to solve the contradiction? One may propose taking the Protocol at its word: despite the clear “anti-GMO” bias, the aforementioned preamble to the treaty intends to regulate the transboundary movement of “living modified organisms” “that may have adverse effects” on biodiversity and on health – take good note of the conditional form. So let’s ask ourselves what these organisms are: among all the recombinant DNA crops currently authorized in various countries, the impact on the environment and health has been studied, restudied and studied yet again, and there is not one cultivar which allows us to imagine negative effects, in one sense or another, which the Protocol wants to safeguard against (Nicolia et al. 2014): indeed, the leading global authority established to monitor our health declares that “GM foods currently traded on the international market have passed risk assessments in several countries and are not likely, nor have been shown, to present risks for human health” (World Health Organization 2005, p. iii and p. 24). Therefore, why should we imagine that the mere handling and transporting of “LMOs” may be dangerous? It would not even be necessary to abolish this treaty; it would be enough (not) to apply it in accordance with its introduction, and leave it there waiting for some pre-authorized crop to show possible risks, in order to apply only at that moment all the measures which it envisages: it waits and hopes, we might say, because when a new product of this type is approved at national or regional level and has already run the gauntlet of very robust analyses, almost by definition its transportation is declared immune from risks.
But it is not so easy. In fact, that clarification by which the Protocol should regard the handling of only potentially harmful LMOs (“that may have adverse effects”) reappears only in Art. 4 (“Scope”) which does nothing more than repeat the words of Art. 1 (“Objective”) but then disappears from all the rest of the treaty, which calmly moves on to impose preventative requirements on the transport of any “LMO”: on all of them, without any more of the preliminary theoretical distinctions. In fact, apparently it is not denied that harmless “LMOs” may exist; a secondary paragraph states: “The advance informed agreement procedure shall not apply to the intentional crossboundary movement of living modified organisms identified in a decision of the Conference of the Parties serving as the meeting of the Parties to this Protocol as being not likely to have adverse effects on the conservation and sustainable use of biological diversity, taking also into account risks to human health.” (Art. 7. Application of the advance informed agreement procedure).
But these extraordinary harmless “LMOs” have not been identified in any agreed list. The question has been considered during some Conferences (http://bch.cbd.int/protocol/cpb_art15/LMOs_traits.shtml#tab=1. Last update 19 August 2014), and a few Parties responded to the invitation to comment on this issue. Some of them, e.g. the USA and the Public Research & Regulation Initiative (PRRI) provide evidence of safety deriving from risk assessments which were conducted at national levels regarding several rDNA crops or traits (e.g. insect resistance); but other government and organizations firmly reiterated a self-evident statement: it is not possible to be completely certain that any “LMO” does not have a hypothetical negative effect. So, instead of deducing that every cultivar must be examined on its own merits, the blanket rejection of “GMO-LMOs” was confirmed as complete and inflexible. The discussion stopped in 2012.
 Besides the basic text of the Protocol, on the official website a certain emphasis is then placed on the “Strategic plan for the Cartagena Protocol on Biosafety for the Period 2011-2020” (http://bch.cbd.int/protocol/issues/cpb_stplan.shtml. Last update 26 April 2016): a series of quite repetitive paragraphs, in which there is constant talk of “Risk assessment and risk management”, “Handling, transport, packaging and identification”, “Transit, contained use, unintentional transboundary movements and emergency measures”. Whoever reads the original treaty and these additional documents without knowing the object of such worries might think that we are talking about powerful poisons, radioactive waste, explosives, bacterial weapons; rather we are talking about sugar beet or soya or cotton… And we are not being ironic and paradoxical. During the preliminary discussions, one of the drafts of the future Cartagena Protocol was presented by the African delegation, which was considered representative of the viewpoint of many poor countries: the text was based on the structure of the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal! (Hobbs, Hobbs and Kerr 2005, p. 287-288) But what are the creators of such hyper-cautious rules afraid of? They are afraid of the fears of others, in a sad but perfect example of the vicious circle: “Why shouldn’t we be wary of this technology and its possible long-term health impacts, if the EU [European Union] is. If it is not good for them, why should it be good for us?” (www.irinnews.org/report/93991/food-rumpus-over-gm-food-aid) With a certain perverse coherence, that is what Tewolde Egziabher, director of the Ethiopian Environmental Protection Agency and regarded as one of the main “architects” of the Protocol, said.
Besides the basic text of the Protocol, on the official website a certain emphasis is then placed on the “Strategic plan for the Cartagena Protocol on Biosafety for the Period 2011-2020” (http://bch.cbd.int/protocol/issues/cpb_stplan.shtml. Last update 26 April 2016): a series of quite repetitive paragraphs, in which there is constant talk of “Risk assessment and risk management”, “Handling, transport, packaging and identification”, “Transit, contained use, unintentional transboundary movements and emergency measures”. Whoever reads the original treaty and these additional documents without knowing the object of such worries might think that we are talking about powerful poisons, radioactive waste, explosives, bacterial weapons; rather we are talking about sugar beet or soya or cotton… And we are not being ironic and paradoxical. During the preliminary discussions, one of the drafts of the future Cartagena Protocol was presented by the African delegation, which was considered representative of the viewpoint of many poor countries: the text was based on the structure of the Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal! (Hobbs, Hobbs and Kerr 2005, p. 287-288) But what are the creators of such hyper-cautious rules afraid of? They are afraid of the fears of others, in a sad but perfect example of the vicious circle: “Why shouldn’t we be wary of this technology and its possible long-term health impacts, if the EU [European Union] is. If it is not good for them, why should it be good for us?” (www.irinnews.org/report/93991/food-rumpus-over-gm-food-aid) With a certain perverse coherence, that is what Tewolde Egziabher, director of the Ethiopian Environmental Protection Agency and regarded as one of the main “architects” of the Protocol, said.
We agree therefore with the double reading of the contorted treaty offered by one of the most important scientific journals, which sees in the degenerate offspring of the excellent mother Convention the result of a strange mix of crypto-protectionism and animosity towards agri-food technological progress: “the agreement has less to do with legitimate concerns about public health or the environment, and more to do with trade protectionism and pandering to anti-technology views.” (Nature 2000, p. 239). All in all, Cartagena “spawned significant regulatory obstacles to the development of GMO-crop technology at great cost to global society and in conflict with many other UN objectives. The suspicion induced by the Protocol is also widely used, overtly or covertly, for political purposes.” (Dubock 2014, p. 210)
Notes:
(1) The Introduction is not part of the text of the Protocol but appears in the official booklet published in English.
(2) Various experts comment on the article; even when they do not openly support dumping of the Protocol, they highlight its inadequacy: www.scidev.net/global/policy/opinion/the-cartagena-protocol-the-debate-goes-on.html (accessed 30 October 2019).
(3) This must not be confused with the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (Convention on Biological Diversity 2010c), a treaty which was also approved in 2010 in the same location and is directly connected to the Convention on Biodiversity.
(4) “We continue to lose biodiversity at a rate never before seen in history – extinction rates may be up to 1,000 times higher than the historical background rate.” (Convention on Biological Diversity 2010a, Message from the Executive Secretary, p. 2)
References:
Adler, Jonathan H. 2000a. More Sorry than Safe: Assessing the Precautionary Principle and the Proposed International Biosafety Protocol. Texas International Law Journal 35:173-205. http://scholarlycommons.law.case.edu/faculty_publications/226
Adler Jonathan H. 2000b. The Cartagena Protocol and Biological Diversity: Biosafe or Biosorry? Georgetown International Environmental Law Review 12:761-777. http://scholarlycommons.law.case.edu/faculty_publications/190
Allen, Kate. 2013. Meet Calestous Juma, Africa’s genetically modified crop ‘optimist’. Toronto Star, June 17. www.thestar.com/news/world/2013/06/17/meet_calestous_juma_africas_genetically_modified_crop_optimist.html
American Phytopathological Society. 2001. Biotechnology and Its Application to Plant Pathology.
www.apsnet.org/members/outreach/ppb/positionstatements/Pages/Biotechnology.aspx
American Society for Cell Biology. 2009. Statement in Support of Research on Genetically Modified Organisms. www.ascb.org/index.php?option=com_content&view=article&id=315&Itemid=31
American Society for Microbiology. 2000. Statement on Genetically Modified Organisms. www.asm.org/index.php?option=com_content&view=article&id=3656&Itemid=341
American Society of Plant Biologists. 2006. Statement on Plant Genetic Engineering.
Ammann, Klaus. 2014. Genomic Misconception: A fresh look at the biosafety of transgenic and conventional crops. A plea for a process agnostic regulation. New Biotechnology 31:1-17.
Arber, Werner. 2010. Genetic engineering compared to natural genetic variations. New Biotechnology 27:517-521.
BASF (2018) Clearfield® Production Systems. https://agriculture.basf.com/us/en/Crop-Protection/Clearfield.html
Berg, Paul. 2008. Meetings that changed the world: Asilomar 1975: DNA modification secured. Nature 455:290-291.
Bernauer, Thomas, and Philipp Aerni. 2008. Trade Conflict Over Genetically Modified Organisms. Pp. 183-193 in Handbook on Trade and the Environment, edited by Kevin P. Gallagher. Cheltenham: Edward Elgar.
Buechle, Kurt. 2001. The Great Global Promise of Genetically Modified Organisms: Overcoming Fear, Misconceptions, and the Cartagena Protocol on Biosafety. Indiana Journal of Global Legal Studies 9:283-324.
Cantley, Mark. 1995. The Regulation of Modern Biotechnology: A Historical and European Perspective: A Case Study in How Societies Cope with New Knowledge in the Last Quarter of the Twentieth Century. Pp. 508-681 in Biotechnology, edited by H.-J. Rehm and G. Reed. Weinheim: Wiley-VCH Verlag.
Colorado State University, Department of Soil and Crop Sciences. 2004. Discontinued Transgenic Products. http://cls.casa.colostate.edu/transgeniccrops/defunct.html
Convention on Biological Diversity. 1992. Convention on Biological Diversity (text of the treaty), Rio de Janeiro: United Nations, 5 June 1992. www.cbd.int/convention/text
Convention on Biological Diversity. 2000. Cartagena Protocol on Biosafety to the Convention on Biological Diversity: text and annexes, Montreal: Secretariat of the Convention on Biological Diversity, 2000. http://bch.cbd.int/database/attachment/?id=10694
Convention on Biological Diversity. 2010a. Global Biodiversity Outlook 3 – Executive Summary. www.cbd.int/gbo3
Convention on Biological Diversity. 2010b. Nagoya – Kuala Lumpur Supplementary Protocol on Liability and Redress. http://bch.cbd.int/protocol/NKL_text.shtml
Convention on Biological Diversity. 2010c. Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization. www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf
Dubock, Adrian. 2014. The politics of Golden Rice. GM Crops & Food 5:210-222.
FAO-IAEA – Food and Agriculture Organization of the United Nations, International Atomic Energy Agency. 2017. Mutant Variety and Genetic Stock (MVGS) Database, http://mvgs.iaea.org
Guruswamy, Lakshman D. 2002. Sustainable Agriculture: Do GMOs Imperil Biosafety? Indiana Journal of Global Legal Studies 9:461-500. www.repository.law.indiana.edu/cgi/viewcontent.cgi?article=1243&context=ijgls
Herring, Ronald J. 2010. Framing the GMO: Epistemic Brokers, Authoritative Knowledge, and Diffusion of Opposition to Biotechnology. Pp. 78-96 in The Diffusion of Social Movements: Actors, Mechanisms, and Political Effects, edited by Kolins Givan, Rebecca, Kenneth M. Roberts, and Sarah H. Soule. Cambridge, Mass.: Cambridge University Press.
Hobbs, Anna L., Jill E. Hobbs, and William A. Kerr. 2005. The Biosafety Protocol: Multilateral Agreement on Protecting the Environment or Protectionist Club? Journal of World Trade 39:281-300.
Hurtado, María Elena. 2013. Ten years in: Taking stock of the biosafety protocol. SciDevNet (website), 28 September. www.scidev.net/global/gm/feature/ten-years-in-taking-stock-of-the-biosafety-protocol.html
ISAAA – International Service for the Acquisition of Agri-biotech Applications. 2019. Brief 54: Global Status of Commercialized Biotech/GM Crops: 2018. Ithaca (NY): ISAAA. www.isaaa.org/resources/publications/briefs/54/
IUCN – International Union for the Conservation of Nature. 2007. Current Knowledge of the Impacts of Genetically Modified Organisms on Biodiversity and Human Health. http://cmsdata.iucn.org/downloads/ip_gmo_09_2007_1_.pdf
Juma, Calestous. 2013. Persecuting Biotechnology. Technology and Policy (website), 11 January 2013. www.belfercenter.org/publication/persecuting-biotechnology
Kerr, William A., Stuart Smyth, Peter W.B. Phillips, and Martin Phillipson. 2014. Conflicting Rules for the International Trade of GM Products: Does International Law Provide a Solution? AgBioForum 17:2. www.agbioforum.org/v17n2/v17n2a02-kerr.htm
Kinderlerer, Julian. 2008. The Cartagena Protocol on Biosafety. Collection of Biosafety Reviews 4:12-65. www.icgeb.org/~bsafesrv/pdffiles/Kinderlerer.pdf
Komen, John. 2012. The emerging international regulatory framework for biotechnology. GM Crops and Food 3:78-84.
National Research Council. 2004. Safety of Genetically Engineered Foods. Washington, DC: National Academy Press.
Nature Biotechnology. 2000. Swapping science for consensus in Montreal (Opinion – editorial). Nature Biotechnology 18:239.
Nicolia, Alessandro, Alberto Manzo, Fabio Veronesi, and Daniele Rosellini. 2014. An overview of the last 10 years of genetically engineered crop safety research. Critical Reviews in Biotechnology 34:77-88.
OECD – Organisation For Economic Co-Operation And Development. 1986. Recombinant DNA safety considerations. Paris: OECD.
Prakash, Channapatna S. et al. 2000-2014. Scientists In Support Of Agricultural Biotechnology (declaration and petition). www.agbioworld.org/declaration/petition/petition.php
Shibata, Akiho. 2013. Introduction. Pp. 1-14 in International Liability Regime for Biodiversity Damage: The Nagoya-Kuala Lumpur Supplementary Protocol, edited by Akiho Shibata. London: Routledge. http://www2.kobe-u.ac.jp/~akihos/en/grenoble_docs/30Shibata_intro2013.pdf
Smith, Frances B. 2000. The Biosafety Protocol: The Real Losers Are the Developing Countries, Washington, DC: National Legal Center for the Public Interest. https://www.heartland.org/publications-resources/publications/the-biosafety-protocol-the-real-losers-are-developing-countries
Tagliabue, Giovanni. 2016a. The Precautionary principle: Its misunderstandings and misuses in relation to “GMOs”. New Biotechnology, 33:437-439.
Tagliabue, Giovanni. 2016b. The necessary “GMO” denialism and scientific consensus, Journal of Science Communication, 15:1-11. http://jcom.sissa.it/archive/15/04/JCOM_1504_2016_Y01
Tagliabue, Giovanni. 2017. Product, not process! Explaining a basic concept in agricultural biotechnologies and food safety. Life Sciences, Society and Policy, 2017, 13:3:1-9. http://rdcu.be/pKBe
United Nations. 1992. Report of the United Nations Conference on Environment and Development, Annex I: Rio Declaration on Environment and Development. www.un.org/documents/ga/conf151/aconf15126-1annex1.htm
Ventura, Arnoldo. 2006. Do we still need the Cartagena Protocol? SciDevNet (website), 12 April. www.scidev.net/global/biotechnology/opinion/do-we-still-need-the-cartagena-protocol.html
WHO -World Health Organization. 2005. Modern food biotechnology, human health and development: An evidence-based study, Geneva: WHO. www.who.int/foodsafety/publications/biotech/biotech_en.pdf
WTO – World Trade Organization. 2006. Reports out on biotech disputes. www.wto.org/english/news_e/news06_e/291r_e.htm
WTO – World Trade Organization. 2010. Dispute DS291 European Communities – Measures Affecting the Approval and Marketing of Biotech Products, up-to-date at 24 February 2010. www.wto.org/english/tratop_e/dispu_e/cases_e/ds291_e.htm
Dr. Giovanni Molteni Tagliabue is an independent researcher based in Italy who studies and reports on the philosophy of life sciences and political science. He specializes in epistemological, socio-political and legislative aspects of agricultural biotechnologies and is the recipient of the 2017 Innoplanta Science Prize.
A version of this article was originally published at European Scientist and has been republished here with permission. European Scientist can be found on Twitter @EuropeScientist































