I run a research lab at the University of Pittsburgh where we focus on developing tools that can control the safety of CRISPR-based gene therapies, with an emphasis on regulating the immune system. The question we ask ourselves, and which many other scientists in the field are also working on, is this: How can we actually engineer safety and controllability mechanisms into CRISPR?
There are a number of different ways to engineer safety, each of them tackling different aspects of the problem. One method is to modify the Cas9 protein, changing its sequence to make it less recognizable to T cells and other components of the immune system. The goal is to avoid inducing an immune overreaction in a person’s body. We also need to control where CRISPR operates, meaning we want to precisely restrict the edit to the targeted organ and reduce its activity in other organs. There are ways to restrict the activity of CRISPR spatially, such as limiting the expression of CRISPR’s GPS-like guide molecule, known as “gRNA,” to certain cells or contexts. We also need to make sure that we don’t accidentally edit germ cells, which would risk the outcome of a gene edit being passed down from one patient to their offspring. Though it is rare, it can happen, and you certainly don’t want it to happen unintentionally. So safety switches that can destroy CRISPR tools if they end up in the wrong cells are something my group and others have been working on.
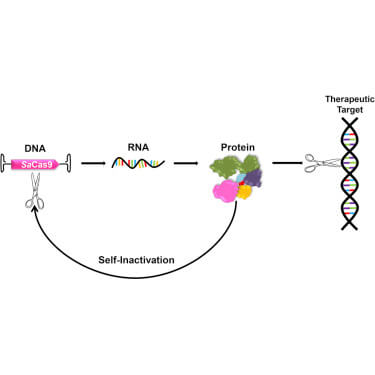
We also need to limit the time CRISPR operates in the cells to the absolute minimum required for the job, so we avoid unintended cuts and alterations to other genes. That can be achieved, for instance, by self-cleaving switches using a self-deleting AAV-CRISPR system, which is work being done by researchers at Rice University and Baylor College of Medicine.
And sometimes, we just want to reduce the footprint of a gene in the cells. For this, we don’t have to permanently disable the gene through cutting, but instead we can epigenetically silence its expression, which doesn’t require a permanent change to the DNA itself. CRISPR variants that target and destroy specific mRNA sequences to reduce gene expression or that allow us to achieve our goal without cutting or altering DNA are safer alternatives for clinical applications. In fact, all of these will help make CRISPR therapies safer, allowing us to translate CRISPR faster to the clinic.

Alongside the application in human therapeutics, CRISPR is being used as a diagnostic tool in humans and animals as well as in agriculture and environmental monitoring. Safety considerations to control CRISPR functions are important for these applications too. For example, the use of CRISPR to engineer gene drives (which are genetic approaches that force the spread of certain traits in the environment through engineered species, such as pathogen-resistant mosquitoes to reduce the spread of malaria). While promising, they have also raised a lot of questions about safety and controllability. So researchers are working on safety mechanisms such as split-CRISPR based gene drives, which are designed from the start to be more controllable and safer to use in the environment.
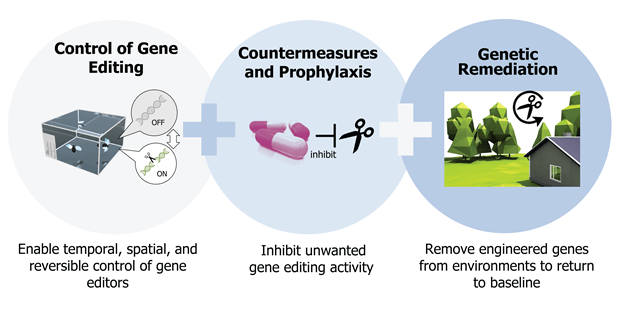
Putting all these pieces together, we have a moral responsibility not to deprive our future generation of these technologies. But we need to be very creative and proactive in controlling the risks. Eventually, I’d like to see even more safety mechanisms or design ideas. These are things that aren’t technically possible yet, like the ability to track whatever DNA we change. That would be ethically thorny to do in human beings—you don’t want to mark people who are genetically modified and thus distinct from society. But it would be beneficial to understand which individual animals, like flies or mosquitoes, are genetically modified.
Or imagine the ability to reverse whatever we change in DNA—to design “kill switches” with the ability to shut down or destroy one of our gene edits at any time, or reverse it back to its unedited state, should an adverse reaction occur. Or imagine even more broadly the ability to limit the expression or activity of CRISPR tools to a time or location we want, whether it’s inside the body or even a particular location in this world. Ideally, we would develop a capacity to dispatch biomolecular countermeasure safety kits to every household.
These are things we want to imagine for a technology like this, that moves fast and spreads fast.
Editor’s note: This article is the result of a conversation former NEO.LIFE editor Brian Bergstein had with Kiani for the book NEO.LIFE: 25 Visions for the Future of Our Species and has been updated to reflect technological developments since its original publication and the evolution of her work.
Samira Kiani is an associate professor of pathology and bioengineering at the University of Pittsburgh, founder of GenexGen, and a film producer. Follow Samira on Twitter @samira_kiani1
A version of this article was posted at Neo.Life and is used here with permission. Check out Neo.Life on Twitter @NEOdotLIFE































