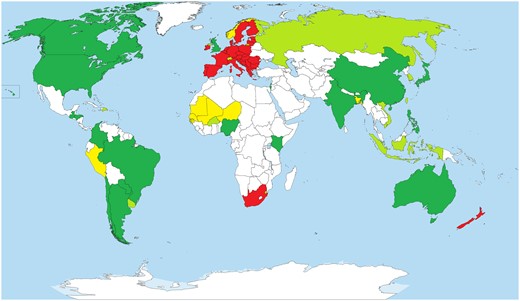
In Switzerland, the association “Varieties for Tomorrow” which is composed of various actors from the Swiss agriculture and food industry called for an open and differentiated approach to NGT in plant breeding. Furthermore, the Science Commission of the States voted for the first time in favor of GEd.
…
In Canada, there is no separate GMO regulation, but there is a regulatory regime dealing with novel foods and with respect to risk assessment obligations with new substances notification.
…
South Africa which was long time undecided on how to deal with GEd has announced its regulatory approaches for “New breeding technologies” through a public notice after a discussion on the GMO status of “NBT products” on October 27, 2021 (Republic of South Africa, 2021). The executive council of South Africa has concluded that for “NBTs” the same risk assessment framework should apply as for GMOs based on the definition of a GMO in the South African GMO Act. Based on this decision, the application forms were updated and products of “NBTs” are seen as a GMO regardless of the type.
In contrast to this, Kenya has recently released its guidelines as well. The Kenyan guidelines rely on a case-by-case evaluation based on the presence of transgenes and are comparable to the ones which are in place in Nigeria including deregulation of cisgenesis and in cases where foreign DNA is absent (“all deletions/knock outs”).