Health care providers may suggest certain supplements for HD patients, based perhaps on a deficiency (vitamins C, B12, E) in the blood, or for general health. But the new findings are different. The researchers didn’t set out to detect a vitamin deficiency, but instead probed the messaging within cells in the HD brain, which led them to a biochemical juncture that revealed the thiamine/biotin connection.
Genetic circuitry
In HD, extra copies of a DNA triplet, in a gene (called Htt) that encodes the protein huntingtin, disturb the brain’s striatum, causing the characteristic uncontrollable movements and mood manifestations. The effects arise both from the repeats in the mRNA transcribed from the expanded gene, and from what that mRNA instructs the cell to produce: extra copies of the amino acid glutamine. Long glutamine strings, called “polyQ,” are common to several brain disorders, including the spinocerebellar ataxias and spinal-bulbar muscular atrophy. (Q is the abbreviation for glutamine.)
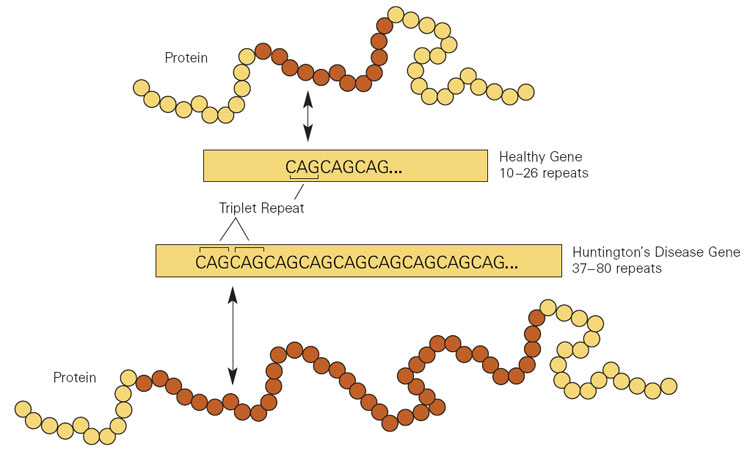
The researchers sought genes that, when mutant or abnormally expressed, affect both the mRNA and polyQ aberrations in the Htt gene behind HD. That led to a class of genes called CPEBs, for “cytoplasmic polyA element binding protein.”
Experiments in flies had shown that CPEBs control part of the Htt mRNAs called the poly A tails. The tails are stretches of the RNA base adenine (A) at the start that stabilize the mRNA, assisting its exit from the nucleus and protecting it from being chewed up by enzymes. Nearly all mRNAs have poly A tails. A second line of evidence implicating CPEB genes is that one of them controls the amounts of other proteins that are altered in the HD brain.

Next, the new experiments.
The researchers assessed the levels of four CPEB mRNAs (aka transcripts) in striatum slices from people who had died of HD and from control brains. And they found triple the level of CPEB1 and half the normal level of CPEB4 in HD brains.
Next, findings in two mouse models echoed the human brain probings. R 6/1 mice, which have the first coding portion of the human Htt gene, exhibited the same CPEB perturbations. A second mouse model that corresponds to people who are “pre-manifest” – they have the mutation but have not yet developed symptoms – showed only the lowering of CPEB4. Perhaps the hike in CPEB1 didn’t happen because these mice don’t live long enough to show symptoms – in rodents that’s the ability to stay upright on a moving tube called a rotarod. These findings mean that CPEB4 levels plunge before CPEB1 levels triple.
When the researchers looked at the genes whose mRNAs the CPEB mRNAs control, they found three with elongated polyA tails – and the expression of this gene trio is also altered in Alzheimer’s disease and Parkinson’s disease. But it was a gene whose mRNA has a shorter polyA tail that brought vitamins unexpectedly into the picture: SLC19A3.
Mutations in SLC19A3 were already known to cause a brain disease that is treatable with biotin and thiamine! Biotin-thiamine-responsive basal ganglia disease (BTBGD) alters the protein that transports thiamine into cells. The condition causes lethargy, irritability, tremors, and spastic and uncontrollable movements, similar to HD. BTBGD arises from direct mutations in the thiamine transporter, whereas HD alters expression of the gene encoding the transporter (how rapidly it is transcribed into mRNA).
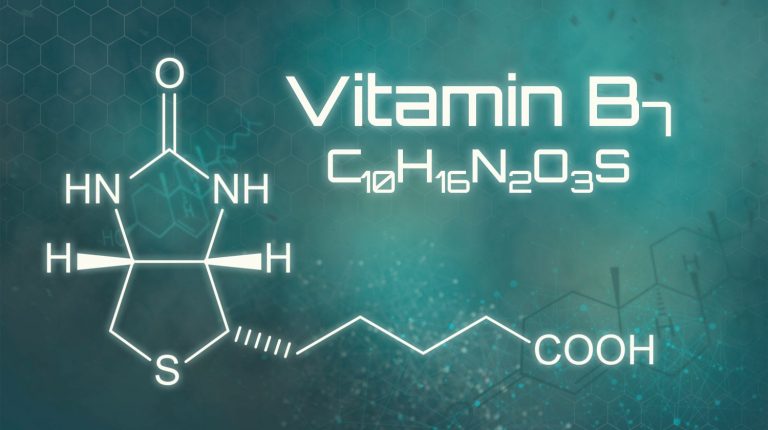
Adding high doses of biotin to the diet of people with BTBGD speeds transcription of SLC19A3, and that increases the levels of the protein that escorts thiamine into cells. But the thiamine deficiency is in the cerebrospinal fluid, not in the blood – perhaps explaining why thiamine levels are ok in the bloodwork of HD patients.
The vitamin connection inspired further experiments. Slices of striatum and cortex from the brains of deceased HD patients showed lower levels of the thiamine transporter. And thiamine was lowered in the cerebrospinal fluid but not in the blood, consistent with BTBGD.
More telling was that HD mice given high doses of biotin and thiamine in their water, starting at 3 weeks of age, no longer fell off the rotarod. And the brains of the pre-manifest mice given the vitamins were okay.
More complicated than popping supplements
On the surface, a vitamin connection to a brain disease that has evaded all attempts at treatment, for decades, is wonderful news. Taking vitamin tablets is cheap, safe, easy to do, and the vitamin is distributed throughout the central nervous system. Plus, high doses of the two vitamins are known to treat a similar disease. Even though pre-manifest individuals and people who already have signs and symptoms of HD are bound to head to the drugstore to stock up on vitamins, the researchers urge caution.
“The doses of these vitamins that led to amelioration of HD-like phenotypes in mouse models are much higher than their recommended daily intake. Besides, it is important to emphasize that, for the highest assessed doses, we observed signs of toxicity in HD mice. This led us to diminish the doses administered to the mice to a point without evident side effects and with measurable positive outcome on HD-like phenotypes,” said corresponding author José J. Lucas, Ph.D, research professor at Centro de Biología Molecular-Severo Ochoa in Madrid, in an email.
So the investigators are conducting an open label pilot clinical trial with a limited number of HD patients, to “analyze whether the high doses of thiamine and biotin required for the therapeutic effect observed in mice are safe and tolerable for patients with Huntington’s disease.” If all looks good, larger trials will follow. Meanwhile, “it is important that patients do not take thiamine and biotin supplements without advice by their neurologist,” warns Lucas.
Until vitamin supplements get an official go-ahead in slowing, treating, or delaying onset of HD, the community of families living with the disease, once again, and perhaps to a great extent than ever, has hope. And that’s priceless.
Thanks to Jane Mervar and Jonathan Monkemeyer for help with this post.
Ricki Lewis has a PhD in genetics and is a science writer and author of several human genetics books. She is an adjunct professor for the Alden March Bioethics Institute at Albany Medical College. Follow her at her website or Twitter @rickilewis
A version of this article was originally posted at PLOS and has been reposted here with permission. PLOS can be found on Twitter @PLOS
This article first appeared on the GLP Aug 18, 2023































