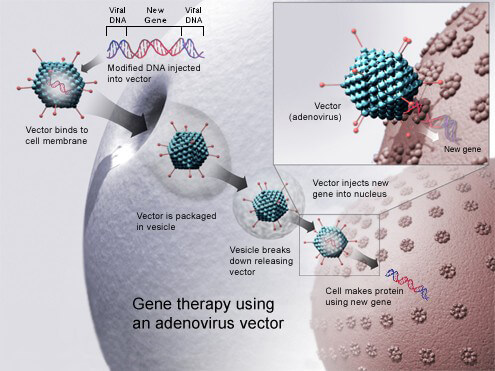
Gene Therapy

Gene therapy treatment restores hearing to five children in China. Will the results last?
After deafness treatment, Yiyi can hear her mother and dance to the music. But why is it so noisy at ...

Treating mental health disorders: Is CRISPR a long-hoped-for silver bullet?
“I was born with the murder gene.” That’s the chilling statement in a true-life story featured in Esquire years ago ...

How gene therapy could eventually snip out diseases in the womb
Recent advances in lab animals may bring medicine closer to achieving it—but this field is still in its infancy ...

Why did Ellie in the Last of Us not succumb to Cordy, the zombie virus? Stem cells might explain it, and that could yield real-life vaccines
It’s unsettling to watch The Last of Us, in which parasitic fungi turn humanity into flesh-eating zombies, just as the ...

Next generation medicine: Will the people who most need gene therapy and gene-editing tools have access to them?
One of greatest risks of gene editing tools ‘is that the people who would benefit most will not be able ...

Million-dollar gene therapies offer salvation for many patients but pose financial challenges for government-funded health care systems
A wave of transformative but hugely expensive treatments is challenging the budgets of health systems in wealthy nations. Now countries ...
Gene therapy delivered directly to the brain treats extremely rare disease in children
When Rylae-Ann Poulin was a year old, she didn’t crawl or babble like other kids her age. A rare genetic ...

Zinc fingers: New AI-powered biotech innovation could offer unique advantages over CRISPR when building gene-editing disease therapies
A new study has developed what the researchers call the "world's first" simple, modifiable proteins. Called "zinc fingers," these special ...
Single shot genetic cures are coming. Will insurance companies pay for these multi-million dollar therapies?
Some of Steven Pipe’s hemophilia patients consider themselves cured. In a trial Pipe led from 2018 to 2021, they received ...

Gene therapy update: More than 2,000 treatments are in development worldwide as revolution spreads to low- and middle-income countries
Gene therapy is at the forefront of modern medicine. By making precise changes to the human genome, these sophisticated technologies ...

Podcast: ‘Regenerative’ farming—a green fad; Gene-edited bacteria destroy tumors; Banana-flavored beer
So-called "regenerative" farming is gaining traction as a method of sustainable food production. Does it live up to the hype ...

Gene therapy approvals now at four with treatments for inherited anemia and degenerative brain condition — but costs are stratospheric. Why?
The FDA recently approved two gene therapies with hefty price tags, the first for an inherited anemia and the second ...

Novel gene therapy study offers hope for definitive colorblindness cure
Researchers from the University College London (UCL) used gene therapy to partly restore the function of the retina's cone receptors ...

$2.8 million Zynteglo gene therapy: Bluebird sets price on one-time beta-thalassemia treatment replacing red blood cell transfusions
Zynteglo has become the first cell-based gene therapy to be approved in the US, getting the nod from the FDA ...

Viewpoint: Shining future promised by gene therapy blocked by researchers who refuse to share data
Transparency in gene therapy research — which can be accomplished without compromising commercial prospects — is vital to success. One ...
Understanding genetic basis for heart disease opens up opportunity for gene editing solution
Later this year, Verve Therapeutics of Cambridge, Ma., will initiate Phase 1 clinical trials to test VERVE-101, a new medication ...
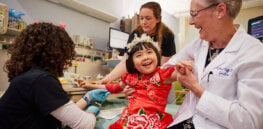
Children with deadly immune disorders remain healthy a decade after being treated with gene therapy
Over a decade ago, UCLA physician-scientists began using a pioneering gene therapy they developed to treat children born with a ...

Increasing yields without genetic modification? A bio-spray that silences plant genes is in the works
The use of bioactive molecules has shown the capability to infiltrate into plant leaves and into the cells themselves with ...

Is there an ‘exercise gene’? Scientists believe CRISPR guided gene therapy could help curb muscle loss in elderly people
“Many millions of elderly people worldwide suffer from sarcopenia, a disease that is characterized by muscle wasting. A large proportion ...

How early failures dogged gene therapy — and why the future looks so much brighter now
Jesse Gelsinger was 18 years old in 1999, when he joined one of the first clinical trials of gene therapy ...
Gene therapy evolves to treat blood cancers and numerous other rare disorders
Gene therapy has come a long way since its first human proof-of-concept trials in the 1990s. The approach—which involves fixing ...
One-and-done intravenous gene editing infusion might eventually be able to cure HIV
In 2014, Temple University researchers proved they could use state-of-the-art molecular scissors to cut out dormant HIV hiding in human ...

mRNA COVID vaccines were decades in the making
In the 1990s and for most of the 2000s, nearly every vaccine company that considered working on mRNA opted to ...

‘It would be huge to see my granddaughter’: In an attempt to cure blindness, scientists use CRISPR to edit DNA inside patients’ bodies
Carlene Knight would love to do things that most people take for granted, such as read books, drive a car, ...

‘Optogenetics’ miracle? Gene therapy and high-tech goggles partially restore sight to man blinded for 40 years
[A new gene therapy to treat blindness] relies on something called optogenetics. The idea is to edit nerve cells collected ...
Is science finally getting gene therapy right in the effort to fight intractable diseases?
Over time, researchers have devised a number of ways to manipulate the genetic activity within cells, often by giving them ...
Infographic: Gene therapy drugs that silence the effects of faulty genes could help tackle Huntington’s and other neurodegenerative diseases
Huntington’s disease (HD) is an inherited condition that causes widespread deterioration in the brain and disrupts thinking, behaviour, emotion and ...