Roche
US approves immunotherapy for breast cancer for first time
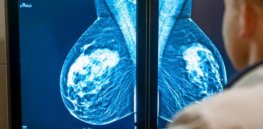
Roche’s cancer immunotherapy Tecentriq (atezolizumab), a PD-L1 inhibitor, scored its fifth Food and Drug Administration approval on [March 8], for advanced triple-negative ...

This small startup wants to change FDA clinical trials by making control groups unnecessary
Could a startup founded by two guys in their 20s change the way medical researchers study patients? The Food and ...

