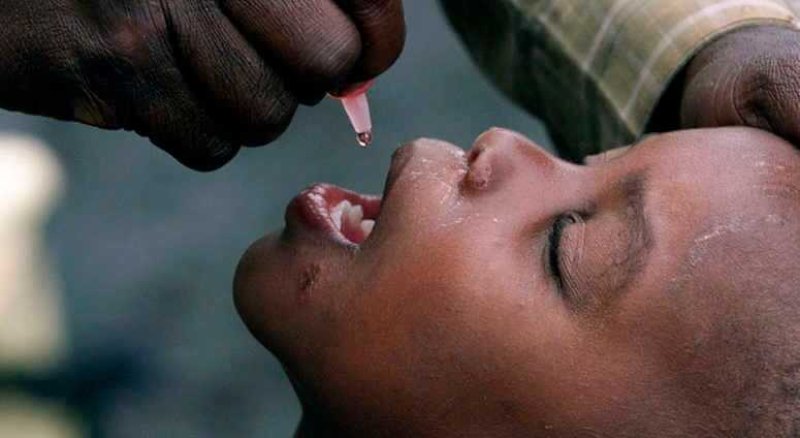
To stem a growing polio crisis, health officials are accelerating the development of a new oral vaccine with plans for emergency approval and deployment in regions with active polio transmission as early as June 2020. The new vaccine, called nOPV2, might conclusively end the outbreaks, caused by the live virus in the vaccine reverting to a virulent form. But expedited approval means skipping the real-world testing of large clinical trials.
…
Oral polio vaccine strains, originally developed by Albert Sabin in the 1950s, can in rare instances revert to virulence, spread, and paralyze children just like polio itself, a phenomenon first recognized in 2000. Because the Sabin vaccine had successfully eradicated wild type 2 poliovirus in 2015, health officials across the world quit administering it the following year. However, herd immunity had not been achieved before the cessation of the type 2 vaccine, which gave an opportunity for un-immunized people to later become infected.
…
nOPV2, the new type 2 oral polio vaccine, has been genetically engineered to avoid the pitfalls of Sabin’s vaccine. The project is funded by the Gates Foundation and coordinated by PATH, a nonprofit developer of public health innovations.
Read full, original post: New Oral Polio Vaccine to Bypass Key Clinical Trials