But had enough evidence demonstrated a fall in the “durability” of the immune response to the first two shots to justify widespread need for a third? And so soon?
The media tended to focus on waning antibody levels, but what about the B and T cells that indicate a more lasting immune response? Then on September 17, a committee of outside experts convened at FDA advised a go-ahead for a booster for people over age 65, with certain medical conditions that put them at elevated risk of serious disease if they contract COVID, and health care workers, at least six months following the second dose. At this writing, matters seem very much in flux.
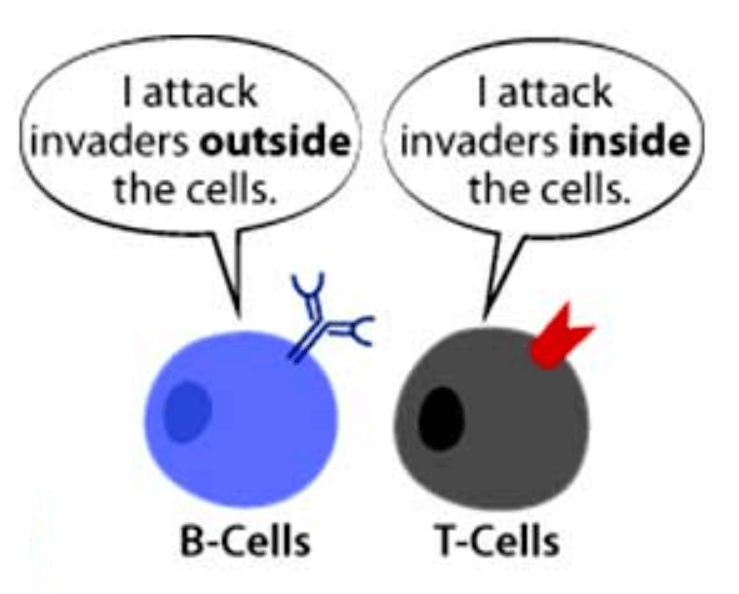
Meanwhile, data are finally coming in on how incredibly effective the original vaccines are, and the news is good. Findings of a trio of recent investigations provide a shot in the arm about the shots in our arms: the approved COVID vaccines stimulate our immune systems to churn out lots of B and T cells – and they last, so far. B cells make antibodies and T cells control much of the immune response and directly attack infected cells.
The three recent papers reveal mounting vaccine-induced immunity. The numbers of people in these studies are much smaller than the thousands in antibody studies, because antibodies are far easier to measure and therefore much more amenable to clinical testing. But charting just antibodies is more a glimpse of the present immune response than looking into the future.
#1 A paper published in Nature June 9, 2021 found that in 25 people who received the Johnson & Johnson/Janssen vaccine, neutralizing antibodies against certain viral variants decreased, but other antibodies and the T cell response remained strong.
#2 A June 28 Nature paper found that after the Pfizer vaccine, B cells persisted in 14 people.
#3 A paper published in Science September 14 reports evidence of “durable protection” from T cells.
Seeking “correlates of protection”
The goal of counting antibodies and T cells is to establish “correlates of protection” – research-backed measurements that indicate defense against an infectious disease. “Defense” may, however, have different definitions: likelihood of being infected, of having symptoms once infected, severe disease, or death.
In pre-COVID times, establishing correlates of protection typically took years, until enough data accrued so that detecting certain levels of antibodies could serve as surrogates for the underlying T cell responses that signal lasting immunity. Even with the supercharged pace of COVID research, we’re not there yet, some experts say.
Tim Stenzel, MD, PhD, director of the Office of In Vitro Diagnostics and Radiological Health at the FDA described how correlates of protection work on a recent CDC webinar. “If a person has antibodies, what does it mean? For COVID, we don’t know what it means enough to determine what an individual patient should do or not do. Just because you have antibodies doesn’t mean you are protected, nor the level of protection.” Getting sick and getting a vaccine both elicit antibodies.
Determining correlates of protection typically takes years and a mountain of data. A classic example is rubella.
“FDA has authorized serology (blood) tests for a level of antibody immunization for rubella. A clinician can see whether or not a patient is above or below, and revaccinate as needed. These are quantitative tests that are linked to international standards that give protection. That is ideally what we’re looking for in studies” of the immune response in COVID, Stenzel said. I had such a test for rubella when I needed proof of immunity for volunteer work and to travel.
But COVID is unlike other infectious diseases in many ways, including the accelerated pace of research. Florian Krammer, PhD, from the Icahn School of Medicine at Mount Sinai, predicts that soon enough evidence will support using levels of neutralizing antibodies to indicate immunity. “A correlate of protection for SARS-CoV-2 vaccines is urgently needed” is the title of a piece he recently published in Nature Medicine, in which he cites several studies that correlate rising levels of antibodies to increased vaccine efficacy.
Counting antibodies or B or T cells aren’t the only ways to assess or infer response to a vaccine on a population level. The recent report from Israel in The New England Journal of Medicine that contributed to the FDA advisory committee’s support for over-65 boosters assessed positive COVID tests and severe illness in 1,137,804 people over age 60 who had Pfizer boosters since July 30, 2021.
The Israeli participants were at least 5 months from the second vaccine dose. Confirmed infection was lower in the booster group by a factor of 11.3 and the rate of severe illness was lower by a factor of 19.5 – seemingly astonishing success. But the authors list important caveats.
Obviously the study didn’t go on for very long, barely a month, and had several confounding factors:
- the effect of the delta variant since the first two shots
- changes in behavior after initial vaccination
- characteristics of the people volunteering for the booster
- not considering co-morbidities
- “detection bias” of people feeling so protected that they didn’t get tested after the booster, hence fewer positives.
Decisions on which vaccines to approve for which age and patient groups awaits further analysis of whether the benefits outweigh specific risks. For example, risk of heart inflammation as a vaccine adverse event may outweigh risk of severe COVID for adolescent males. But in 80-year-olds, the benefit is clear.
In August 2020, Genetic Literacy Project published ‘Vaccine Durability’: COVID-19 immunizations coming soon but will they last? It provided a primer on the components of the immune response – in short, it’s not all about the antibodies. We’re rerunning part of it below, because it’s more relevant than ever:
To be successful, a vaccine’s protection must last or booster shots periodically restore it. Some vaccines lose efficacy over time, including those for yellow fever, pertussis, and of course influenza.
For some vaccines, antibodies and the B cells that make them persist and protect for a long time. For other infectious diseases, like TB and malaria, T cells are needed from vaccines too. B and T cells (lymphocytes) are types of white blood cells, which are part of the immune system.
Antibody response may be ephemeral
“Give a man a fish and you feed him for a day. Teach him how to fish and you feed him for a lifetime,” said Chinese philosopher Lao Tzu, founder of Taoism.
Tzu might have been referring metaphorically to the immune system’s response to viral infection: an initial rush of antibodies that fades as a longer-lasting cell-based memory builds. That memory primes the body to rapidly release antibodies upon a future encounter with the pathogen.
Antibodies are proteins, so they don’t make more of themselves as cells might, and their levels fall. But that doesn’t necessarily mean a person is no longer protected.
To remain effective, a vaccine must mimic the memory component of an immune response, which arises from B and T cells and is therefore called the “cellular” immune response. The shorter-term release of antibodies into the bloodstream is the “humoral” immune response (“humor” means fluid).
A strong antibody response to a vaccine may be a harbinger of lasting B and T cell protection, but vaccines may be marketed before their durability is known – a complete understanding of how long a vaccine’s protection lasts can take years. The vaccine against the mumps, for example, went on the market in 1967, but in 2006, several colleges had outbreaks, among students whose childhood mumps vaccine had worn off. A booster extends the coverage.
Clues to a COVID-19 vaccine’s durability come from natural immunity from past coronavirus infections. The antibody response to SARS and MERS persisted less than a year. But so far, the cellular immune response to SARS, the older of the two, has lasted eleven years. It will be interesting to see how the T cell response to SARS-CoV-2 tracks over the years to come.
Clinical trials to evaluate COVID-19 vaccines in people consider both antibody production and the building of cellular immunity. And a vaccine can be even more protective than natural immunity.
“A vaccine elicits memory B and T cells so the immune system remembers how to fight the disease in the future. Natural infection is not likely to produce durable immunity and vaccination will be essential to produce herd immunity to reduce the probability of viral transmission,” said Arlene Sharpe MD PhD co-director of the Evergrande Center for Immunologic Diseases at Harvard Medical School and Brigham and Women’s Hospital on a recent zoom that MassCPR, a group of Boston-area institutions that formed in early March in response to the pandemic, held. (A year ago it hadn’t occurred to many of us that vaccine hesitancy would counter herd immunity to the degree that it has.)
Making antibodies
The immune system consists of billions of cells and their secretions that can attack newly-encountered pathogens, remember old ones, and recognize “self,” protecting the body’s tissues. The cells travel in the clear lymph fluid, passing through lymph nodes that filter out debris, like swimming pool water circulating through a filter.
The immune system reacts in three stages. First, physical barriers keep pathogens out: skin, earwax, waving cilia in the throat, stomach acid, diarrhea. Next, innate immunity unleashes a bath of inflammatory molecules that are a generalized response to infection.
Finally comes adaptive immunity, which is specific and provides the ‘memory’ that a vaccine emulates. In addition to T and B cells, innate immunity includes the wandering, blobby macrophages, which engulf pathogens and are festooned with bits of a pathogen’s surface – antigens – that alert other immune defenses.
Antibody production begins when a stimulated B cell divides in the bone marrow, giving rise to two types of cells. One, a plasma cell, has a clear oblong area that is a ginormous Golgi apparatus, which processes 2,000 antibodies per second that enter the circulation.
The second “daughter cell” of a dividing B cell is a memory B cell. Like the name suggests, a memory B cell hangs around, and if the pathogen shows up again, jumps into action and pumps out more antibodies, swiftly cutting off the new infection.
An important part of the antibody response is that it’s “polyclonal” – differently-shaped antibodies are produced, each recognizing and binding to a different part of a pathogen, like using different weapons to tackle different parts of an enemy’s body. Some antibodies just bind to a pathogen, but others “neutralize” it, and those are the ones that make a vaccine or immune response effective.
T cells call the shots
T cells come in several varieties and exert complex effects.
- Helper (aka CD4) T cells activate B cells and release interleukins, which also stimulate B cells.
- Killer (aka cytotoxic or CD8) T cells directly attack cells stuffed with viruses, which antibodies can’t do.
- Regulatory T cells (T regs) check the entire immune response so it doesn’t destroy healthy cells. An overactive immune response, deployed against “self” cell surfaces, lies behind autoimmune diseases.
Tracking T cells is important in evaluating potential vaccine durability.
“Investigators are reporting the antibody response in humans infected with COVID who recover tends to drop relatively quickly. To some people that’s an alarm bell and they guess that a vaccine will show little durability. But following recovery from an acute infection, a decline in antibodies is normal B cell biology and is exactly what we predict,” said Daniel Barouch, MD, PhD, professor of medicine, Harvard Medical School and director, Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center. Barouch is lead author of the new paper tracking the T cell response to the Johnson & Johnson vaccine.
So far the COVID vaccines have been a spectacular success. Let’s allow the science to dictate when boosters are needed – and that means not just antibody and case counts. Stay tuned…
Ricki Lewis has a PhD in genetics and is a science writer and author of several human genetics books. She is an adjunct professor for the Alden March Bioethics Institute at Albany Medical College. Follow her at her website or Twitter @rickilewis