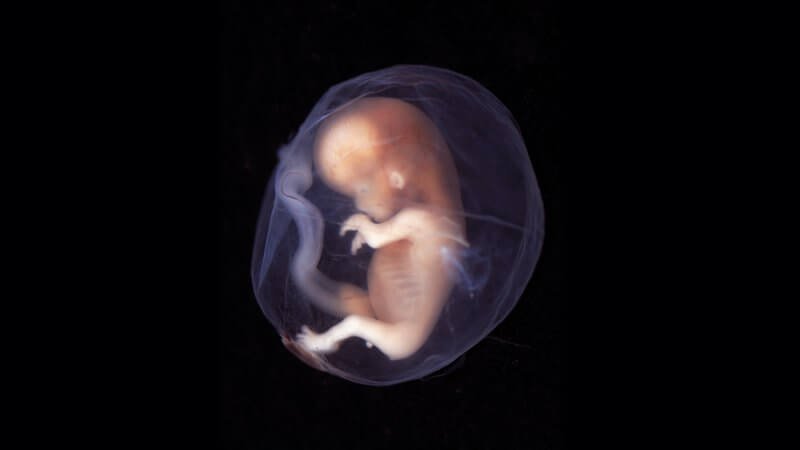
A report in Nature from researchers in the UK and Germany provides an unprecedented view into the early human embryo – thanks to a woman who donated one after having an abortion. She donated through the Human Developmental Biology Resource, which provides automatic bioethical approval from the Institute of Human Genetics, Newcastle and the Institute of Child Health, London.
Probing the earliest of embryos can provide clues to how they are naturally lost in miscarriages, which can be so early as to appear to be infertility, as well as provide clues to how development veers from normalcy as birth defects arise. Substitutes for using human early embryos – like animal models or growing organoids from stem cells – are poor stand-ins.
The first two weeks of embryohood unfold
A tool called the Carnegie stages lists day-by-day changes in vertebrate embryos. For humans, the “period of the embryo” is officially up until day 60 after fertilization (conception). After that, once all structures have been laid down in some form, the prenatal human officially becomes a fetus. Many news reports, politicians, and people supporting the restriction of reproductive rights, erroneously call embryos fetuses.
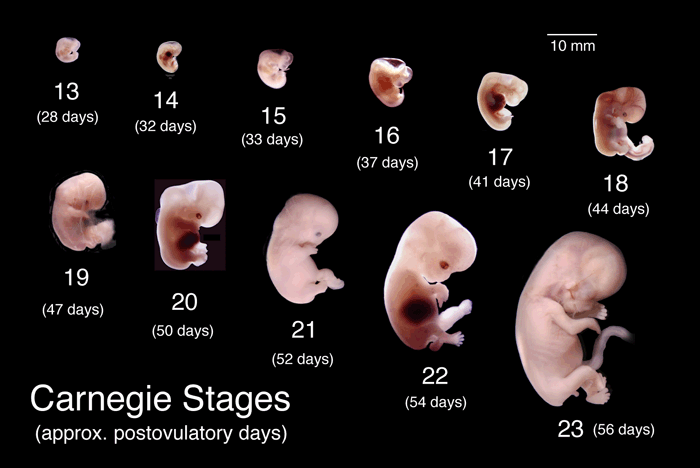
Human prenatal development begins, of course, with a fertilized ovum. That one initial cell divides to yield two, then two to four, four to eight, and so on until the embryo is a solid ball of cells, called a morula (Latin for “mulberry,” which it resembles). It’s now four days from conception, and the embryo is traveling down a tube to the uterus.
On day 5, the ball hollows out as it reaches the rich lining of the uterus and begins to nestle in, by day 7. Soon after, cells are set aside that will become the extra-embryonic membranes, while other cells collect and cling to the inside surface (the inner cell mass), on the way to becoming what biologists call “the embryo proper.”
The inner cell mass continues to lengthen and then curve into a structure called an epiblast which, by day 19, gives rise to the three primordial germ layers. Location is everything in the 3-layered embryo.
Cells on the outside, or ectoderm, will become skin and its derivatives like hair and nails; nerves; tooth enamel and lenses, and the pituitary gland and the inside of the adrenal glands. The innermost cells are endoderm, destined to become liver or pancreas or linings of the respiratory, digestive, and urinary tracts. Everything else – including the muscles and connective tissues that form the bulk of the body – comes from the middle layer, or mesoderm.
Revelations from the embryo
Richard Tyser, Shankar Srinivas, and their colleagues used single-cell RNA sequencing – aka RNA-Seq – to analyze which genes are transcribed into mRNA and translated into proteins in each of 1,195 cells from the gastrulating embryo. The embryo’s cells had 46 pairs of chromosomes, the telltale Y indicating that it was male.
By analyzing the mRNAs cell-by-cell, the researchers were able to paint a portrait of events in the gastrula in an unprecedented way. They also used standard markers to highlight specific body parts, data on cell locations within the embryo, and a statistical method that groups cells by which sets of genes they are transcribing into mRNA.
The News and Views feature accompanying the research report had a perfect headline: “A peek into the black box of human embryology.” RNAseq’s revelation of gene action at a single-cell level fleshes out function, which isn’t always clear from structure. The experiments revealed several aspects of the embryo.
- The ectoderm that’s part of the embryo clings to similar cells that are part of the amniotic lining, which doesn’t happen in mouse embryos – possibly important for designing experiments that use mice as developmental stand-ins for humans.
- The early embryo showed no sign whatsoever of anything resembling a nerve cell. That happens later.
- The embryo’s future sperm cells are already set aside by two weeks.
- Precursors of blood cells appear as colored dots in the yolk sac.
Another important finding gleaned from this one donated embryo has to do with the controversial human embryonic stem (hES) cells. Some people, particularly religious fundamentalists, have objected to the use of these cells, considering them to be cells from human embryos. But they are not and never were. Rather, hES cells are cultured in glassware from cells of an inner cell mass, from an embryo.
Because of this oft-misunderstood origin, researchers were never quite sure exactly which cells hES cells correspond to in an actual human embryo. But the RNAseq of the donated embryo makes clear that hES cells are equivalent to cells from the embryo before it implants in the uterus – before the fifth day after conception. They are NOT the equivalent of the day 19 gastrula. A hES cell requires a brew of growth and transcription factors to specialize sufficiently to equal the state of a gastrula that is on the brink of unfurling into primordial organs.
Shortly after the gastrula paper was published, a similar report covering a week earlier in human prenatal development appeared in Nature, from researchers in France, Austria, and Belgium. They used “IVF extras” donated in France to culture blastoids, which are lab-nurtured to resemble the blastula, the hollow ball of cells that appears 5 to 7 days after conception. At the end of this critical time, the embryo nestles into the uterine lining as the pregnancy hormone hCG is made. RNAseq on the blastoids revealed initial expression of the genes that guide the specialization of the three layers of the embryo into the gastrula.
It’s worth noting that these two papers, which provide peeks into the prenatal period that will inform development of contraceptives and improve understanding of infertility and early pregnancy loss, come from Europe, with routine informed consent from the donors of the biological material.
Glimpsing the prenatal period
Today it’s easy to view images of prenatal humans with Google, but when the public first saw such things on the cover and 16 pages of Life Magazine in 1965, there was shock and awe.
Swedish photojournalist Lennart Nilsson took those iconic photographs, and published even more of them in a now-famous book, A Child is Born, that year. He’d first seen a human fetus in 1952, on assignment at Sabbatsberg hospital in Stockholm. The fetus floated in a glass jar, preserved in formaldehyde. Nilsson, about to become a father, was riveted, and brought the jar home to photograph, publishing the first images later that year.
I, too, have seen a prenatal human floating in formaldehyde, but it was in a test tube. Preserved at 44 days of gestation, the embryo is about the size of a thumbnail. I keep the test tube, wrapped up in gauze, in my desk drawer.

There is a story behind this. I didn’t intentionally take the embryo.
In 1990, I was teaching genetics at a state university. My department had a collection of human embryos and fetuses that an obstetrician had donated in the 1950s, collected from patients who had miscarried. I’d wanted to show them to my class to illustrate human prenatal development.
The smallest embryo was the size of a pea; the largest fetus, at 8 months, was stuffed into a large Hellman’s mayonnaise jar, disturbingly baby-like. One day I’d inadvertently brought my daughter Sarah with me during the time I had the tubes and bottles, and when she saw the mayonnaise jar and its contents, she burst into tears.
A few days later, without Sarah, I wheeled the collection across campus in a grocery cart. A reporter for the school newspaper stopped me, assuming I was displaying an anti-abortion exhibit. I explained that I was not, it was simply a teaching tool.
Class went well. But back in my office, when I packed up the collection, a lone test tube somehow fell into my desk drawer. I returned the tubes and bottles, and found the missing test tube years later, when I cleaned out my desk.
I had an earlier experience with viewing human embryos and fetuses, when I was between Sarah’s age of 6 and my age as the young professor.
As a senior in high school, I had worked in a lab that processed the remnants of abortions. It was during the pre-Roe v. Wade spring of 1972, and pregnancy termination as a medical procedure was legal in New York state. Thousands of women traveled there for that purpose, which is why I got the job.
The bloody tissue arrived at the lab in small, clear cups, like fruit cocktail. Being a budding biologist, I’d stared in fascination at the cups, swishing the contents, trying to identify tissues and bits of organs, oblivious to the heartache that likely led to the decision to end the pregnancy.
This research has spurred me to reflect on my past experience, the significance of this research, and what lies ahead.
I applaud the woman in the UK and the couples at the Assisted Reproductive Technology unit of University Hospital, Nantes, France, who donated their early embryos to enable researchers to probe how cells sort themselves into a functioning whole. If the embryos were to be discarded as medical waste, why NOT allow researchers to analyze them? The new knowledge can help couples struggling with early pregnancy loss and what appears to be infertility, and reveal the beginnings of some birth defects.
I think that it is possible to revere and respect prenatal humans, and also to learn from those that are no longer living, to help those who are.
Ricki Lewis has a PhD in genetics and is a science writer and author of several human genetics books. She is an adjunct professor for the Alden March Bioethics Institute at Albany Medical College. Follow her at her website or Twitter @rickilewis