In March of 2021, researchers at the University of Tokyo announced that they had successfully grown steak in a lab. The cultured meat mimics real muscle and contracts when stimulated with electricity. The team wasere only able to generate small pieces of meat and have yet to gain ethical approval to “taste-test” the samples. However, this finding still represents a major breakthrough in the mission to harness the latest scientific technologies and produce more sustainable meat.
The idea of growing consumable meat in a lab has been around since 2013, when researchers in London showcased the world’s first lab-grown burger. Produced from billions of cow cells and costing over $330,000 o make, the first “slaughter-free” beef burger certainly turned heads and left many wondering if it was a viable concept at all. Those who tasted it claimed it was pretty close to the real thing but, at such a high cost, was it really worth the effort?

Fast-forward eight years and we now find ourselves in a position where companies in 16 countries are developing lab-grown meat products. One such company made headlines recently by gaining approval to start selling their “chicken bites” in Singapore; presumably for less than $330,000 each. So how do you go from a collection of cells in a lab dish to a quarter pounder burger? Will it taste the same? And how long before we start to see such products in our local store?
Making the (lab) meat
Let’s start with the process. If we take beef as an example, scientists start by taking a small sample of muscle from a cow. Stem cells are then extracted from the tissue and grown in a lab dish, where they rapidly expand into millions of muscle cells. The cells then organize into fiber which subsequently arrange into a structure akin to the muscle tissue found in meat. It is, however, not as simple as just growing lots of cells in a dish. You need a third element.
Every tissue in the body is comprised of a mixture of cells and materials (sugars, proteins, etc.). This is true for every species on the planet. If you want to grow muscle tissue in a lab, you need to have something that represents the 3D network of materials that the cells use as a “scaffolding” around which to build new tissue. Without it, you are doing the equivalent of trying to assemble a car with just wheels and an engine.
Lab-grown meat is no exception to this rule. If you simply extract cells from muscle tissue and grow them in a dish you will not be able to create a piece of meat no matter how long you wait. The 3D scaffold provides the means to build the muscle tissue. In the case of the Japanese study mentioned earlier, the researchers developed an intricate scaffold comprised of many materials commonly found in tissue. When embedded in the scaffold, cells that were extracted from cow muscle aligned and formed muscle fiber. The result was a structure that, according to lead author Mai Furuhashi, could “mimic the texture and mouthfeel of steak”.
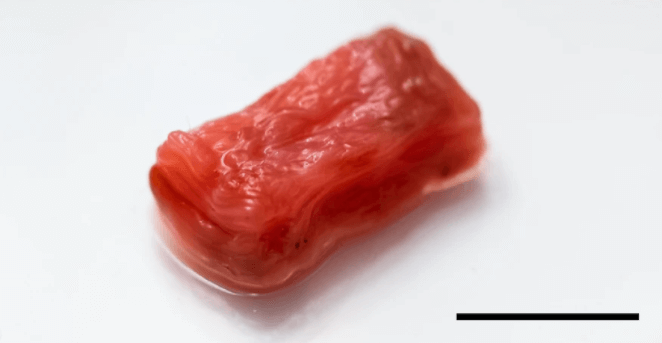
This technique is, therefore, a promising option but it is heavily reliant on using materials that are expensive and still require some form of animal slaughter. Collagen was one such material used in the study; commonly obtained post mortem from rats, pigs or calves. The idea of lab-grown meat is to remove the slaughter to address the ethical and environmental issues with factory farming. Reducing the reliance on animal products (aside from cells and growth media) as much as possible is inevitably the goal here.
Pivoting from slaughter, thanks to plants
Fortunately, researchers at Boston College may have a solution. Instead of growing the extracted cells in a scaffold of animal proteins, they have turned to plants. Complex organisms are not the only lifeforms made up of proteins, fats and sugars. Plants are too, and the materials play the same roles in allowing plant cells to form 3D structures. The team harnessed this by using de-cellularised spinach leaves as the scaffold for cow muscle cells. Cells are removed from the spinach, leaving behind a network of tubules similar to the blood vessels found in meat. When grown on the spinach “skeleton,” cow cells started to differentiate into muscle mass. The work is still early in development but Glenn Gaudette, the lead author on the study, believes we have a lot to gain in looking towards plants as a way of cultivating animal products.
“We need to scale this up by growing more cells on the leaves to create a thicker steak,” he said. “In addition, we are looking at other vegetables and other animal and fish cells.”
The Boston team isn’t the only ones looking towards plants for a suitable scaffold to generate meat in the lab. Researchers in Israel are utilising textured soy protein to grow muscle cells. The technique, originally designed for medical applications such as human tissue transplants, allows the generation of cultured meat tissue in 3-4 weeks. It carries the advantage of utilizing a product that is inexpensive and a readily available by-product of soy oil production. Shulamit Levenberg, a member of the Israeli team, had the following to say:
“The current research using soy protein is important in proving the feasibility of producing meat from several types of cells on plant-based platforms, which increases its similarity to conventional bovine meat.”
But, will people eat it?
So it seems the technology may be already in late-stage development to take lab grown meat to the next level. It is, however, all a bit moot if the product doesn’t taste or feel right. One of the biggest revolutions in food over the last decade has been the development of plant based meat alternatives. While they can replicate some of the flavours of meat, the texture and overall palate is usually distinguishable from the real thing.
Do we risk having the same issues with lab-grown meat? It is hard to say at the moment. Very few have had the opportunity to taste lab-grown meat. Those that have comment on its “meaty flavour” but note differences to the real thing. Ultimately, we may have to accept a change in flavour. It is increasingly clear that our reliance on factory livestock farming is becoming further removed from the current ethical climate. Moreover, meat farms are listed as a significant contributor to our global greenhouse gas emissions, leading to increased pressure on the meat industry to find more sustainable ways of producing food. Lab grown meat has the potential to solve some of these issues by removing the need for the acres of land and thousands of livestock that many farms currently require in order to supply the demand for meat.
So finally, perhaps the biggest question. When will we see lab grown meat in our local stores? We need to scale up production enough to match our immense demand. Some experts predict we could start seeing products on sale within the next five years. It is, however, a little difficult to see that at the moment. We might start seeing lab-grown chicken nuggets but creating un-processed, prime rib eye steak will be a lot more challenging. Current studies are only reporting production of pieces weighing a few grams (~0.1 oz.). The big challenge is scaling it up to producing a 10 oz steak, without it costing as much as a house in the suburbs of Los Angeles. Production on such a wide scale in a cost-effective manner will take some time. The biggest question is, when we get there, will you be choosing lab grown over grass fed?
Sam Moxon has a PhD in tissue engineering and is currently a research fellow in the field of regenerative medicine. He is a freelance writer with an interest in the development of new technologies to enhance medical therapies. Follow him on Twitter @DrSamMoxon































