 Most biotechnologies evolve over three decades or so, but the idea of gene therapy has been around since the late 1950s, blooming soon after Watson and Crick solved the structure of DNA. When my book The Forever Fix: Gene Therapy and the Boy Who Saved It was published a decade ago, it would still be 5 years before the first approval. That treatment, the subject of my book, enabled the blind to see, sometimes in just days.
Most biotechnologies evolve over three decades or so, but the idea of gene therapy has been around since the late 1950s, blooming soon after Watson and Crick solved the structure of DNA. When my book The Forever Fix: Gene Therapy and the Boy Who Saved It was published a decade ago, it would still be 5 years before the first approval. That treatment, the subject of my book, enabled the blind to see, sometimes in just days.
Why has the pace of gene therapy been so slow? Cost is one barrier. Other concerns are the degree to which a gene therapy actually helps, how long the effect lasts, and what proportion of patients respond.
A short list
FDA’s gene therapy roster is here, but a caveat is necessary.
The list lumps gene therapy in with cell therapy, inviting unintentional hype from media folks unfamiliar with the science. Most entries actually refer to using stem cells to treat blood cancers and related conditions. An example: cartilage cells are sampled from a person with a bum knee, mass-produced in a dish, and then injected into the knee, where they fuel production of more cartilage.
My favorite example of not-really-gene-therapy on the FDA’s list targets facial wrinkles, also using patients’ lab-expanded cells: 18 million fibroblasts injected three times churn out collagen, filling in the offending skin craters.
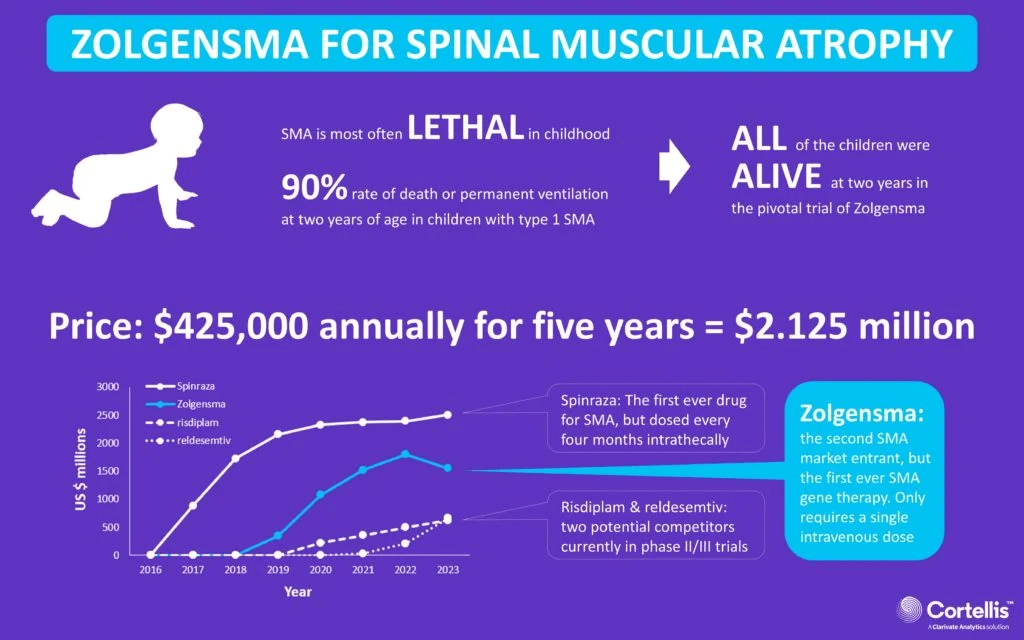
Buried in the FDA’s list are the first two actual gene therapy approvals. Luxturna (Spark Therapeutics) treats RPE65 mutation-associated retinal dystrophy and has restored vision in many patients since its approval at the end of 2017. The second approved gene therapy, in 2019, is Zolgensma, to treat spinal muscular atrophy, from Novartis Gene Therapies.
The two newbies
FDA approved Zynteglo on August 17, aka betibeglogene autotemcel or eli-cel. It treats the blood disorder beta thalassemia, which causes weakness, dizziness, fatigue, and bone problems. People with severe cases need transfusions of red blood cells every two to five weeks, which can lead to dangerous buildup of iron.
Zynteglo is a one-time infusion of stem cells descended from a patient’s bone marrow in which functional beta globin genes have been introduced aboard lentiviruses – disabled HIV. The $2.8 million treatment is approved for adults and children.
Two clinical trials enrolled 91 patients, 36 of whom improved enough to no longer need transfusions. Bluebird estimates that 1,300 to 1,500 people in the U.S. may be candidates for Zynteglo.
The second go-ahead is for Skysona, approved September 16 for “early active” cerebral adrenoleukodystropy (CALD). The condition destroys the protective myelin sheath around brain neurons.
A stem cell transplant can cure CALD. Skysona is for the 700 or so boys aged 4 to 17 who can’t find matched donors. Nearly fifty percent of them die within five years of symptom onset.
But like many gene therapies, Skysona isn’t a magic bullet. In the two ongoing clinical trials, the metric for assessing improvement is slowing neurologic decline, tracking “major functional disabilities.” These include loss of communication skills, vision, and of voluntary movement, which impairs mobility, eating, and urinary retention.

The 2-year study that led to the FDA approval followed boys with mild or no symptoms, diagnosis possible early due to newborn screening in many states. Those who received Skysona had a 72% likelihood of survival over the two years without developing new major functional disabilities, compared to 43% among untreated boys. The trial will follow participants for 15 years. Since many states are now screening newborns for ALD, perhaps boys destined to develop symptoms can receive Skysona before that – if someone will pick up the $3 million tab per patient.
The promise of “lifelong cures” stokes pricing
Gene therapy companies have long justified high costs with the expense of the bench-to-bedside trajectory. So I was surprised to see a new study published in JAMA Network Open, “Association of Research and Development Investments With Treatment Costs for New Drugs Approved From 2009 to 2018,” finding none. The authors admonish companies to “make further data available to support their claims that high drug prices are needed to recover research and development investments, if they are to continue to use this argument to justify high prices.”
Because the paper uses terms like “first-in-class,” “accelerated approval,” “breakthrough therapy,” “orphan,” and “priority review” – language I’ve often seen attached to descriptions of gene therapy – I assumed it would include Luxturna, which costs $850,000 for both eyes. But the new report omits drug names, instead citing a 2020 paper from the team that did. No Luxturna. That’s probably because the researchers evaluated R&D costs only for products with publicly available data – that’s 63 drugs, a mere fifth of new approvals. The new report, of course sent out in news release form to the media, provides more a glimpse than a revelation.
So perhaps gene therapy is an exception for which high prices are indeed required to recoup investment. A viral vector to deliver DNA can cost $500,000 or more to produce, let alone engineer and develop.
Companies also use the one-and-done strategy to justify high prices. The homepage of bluebird bio’s website, for example, proclaims “we’re pursuing curative gene therapies,” although the data on Skysona for CALD indicate incremental change. Axios reports on how Medicaid, private insurers, and companies will help address cost concerns.
Gene and genome editing: Safety and precision improve
While bluebird bio bats around the “c” word – cure – it also introduces a long-needed granularity to the terminology. The company has replaced “gene therapy” with the more accurate “gene addition therapy.” That’s what the four approved gene therapies actually do – add working copies of genes, not fixing them in place. Gene therapy is a little like patching a flat tire, not replacing it.
But the next stage of the evolving technology will in fact be fixing genes, courtesy of gene and genome editing. This more precise strategy circumvents the problem of a piece of DNA inserting willy-nilly into a chromosome, perhaps disrupting a cancer-causing gene.
Gene editing with CRISPR has now been around for a decade. The components of the toolkit have been refined to minimize so-called off-target effects that can harpoon unintended genes.
A team at St. Jude Children’s Research Hospital has developed what hematologist Yong Cheng terms “the Google Maps of editing the genome. We provide a new approach to identify places to safely integrate a gene cassette. We created step-by-step directions to find safe harbor sites in specific tissues.” The recipe is published in Genome Biology and the tool available here.
The approach is seemingly simple. Using data from the 1000 Genomes Project, the tool identifies parts of the genome that often bear inserted or deleted DNA sequences among healthy people (and therefore are harmless) and are highly variable. These are the places where unwound DNA loops about itself when replicating just before a cell divides, and could tolerate a healing gene harpoon going astray.
“Safe gene therapy requires two things. Number one, maintaining high expression of the new gene. And number two, the integration needs to have minimal effects on the normal human genome,” Cheng said.
Coda
Gene addition therapy and gene/genome editing are slowly taking their places among other weapons against genetic disease. These include antisense treatments that glom onto mutant genes, small molecule-based drugs, repurposing existing drugs, supplements, and perhaps most important, the therapies that impact life on a daily basis. And so the toolbox expands to tackle the errors in our genes.
Ricki Lewis has a PhD in genetics and is a science writer and author of several human genetics books. She is an adjunct professor for the Alden March Bioethics Institute at Albany Medical College. Follow her at her website or Twitter @rickilewis
A version of this article originally appeared at PLOS and is reposted here with permission. Find PLOS on Twitter @PLOS































