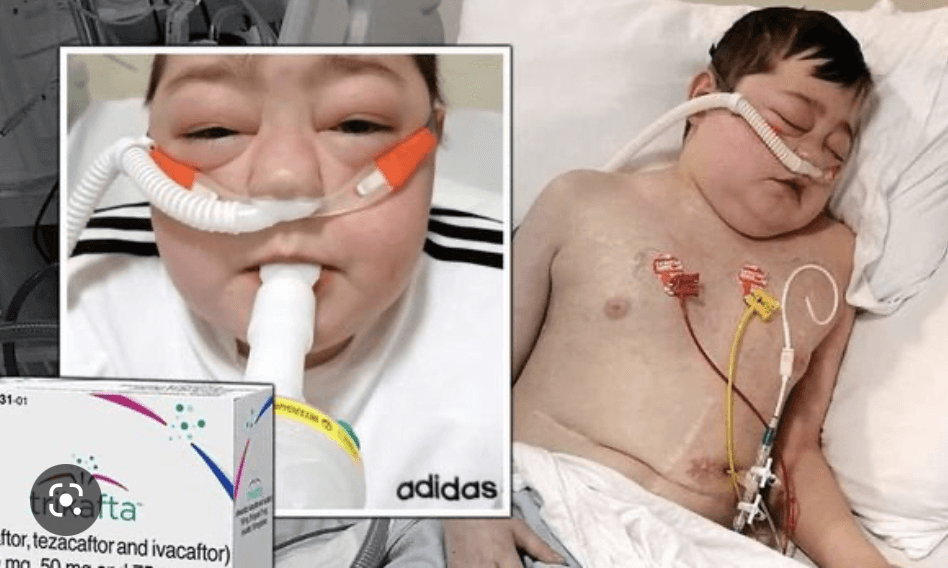
Is cystic fibrosis (CF) a death sentence? It can be for many if it is not treated aggressively and early. It’s the most common fatal disease in many countries, and the most common genetic disease among whites. The genetic disorder effects the lungs and carries a life expectancy of ~46 years.
The battle to contain the disease raises two provocative questions. Why hasn’t natural selection removed the deadly mutation that triggers the disease from the human genome? Are their treatments in the wings that might offer hope to this deadliest of diseases?
New treatments
In January, Maryland-based drug development company Advanced Phage Therapeutics (APT) dosed its first patients in an early-stage clinical trial for a new. It is the result of a mutation in one gene, the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene, which instructs lung cells to produce a CFTR protein that aids in the transport of water in and out of the cells of the lungs in healthy individuals.
In CF patients, this protein is defective, resulting in the build-up of sticky mucus in the lungs that blocks airways and traps antigens like bacteria and viruses leading to recurrent infections and severe lung damage. Additionally, this mutation prevents digestive enzymes from reaching the intestines which negatively impacts on digestion.
This new treatment targets one of the main causes of serious complications in CF; the recurrent bacterial infections of the lungs. Over time patients experience chronic levels of damage to their lung tissue and, sadly, this often culminates in total respiratory failure and death. APT hope to address this by turning to a form of treatment called phage therapy. It is based on a 1917 discovery by French Canadian biologist Félix d’Hérelle which uses bacteria-targeting viruses called bacteriophages to destroy harmful bacteria in the body. In this case, the drug targets and destroys bacterial strains that can cause fatal lung damage in CF patients.
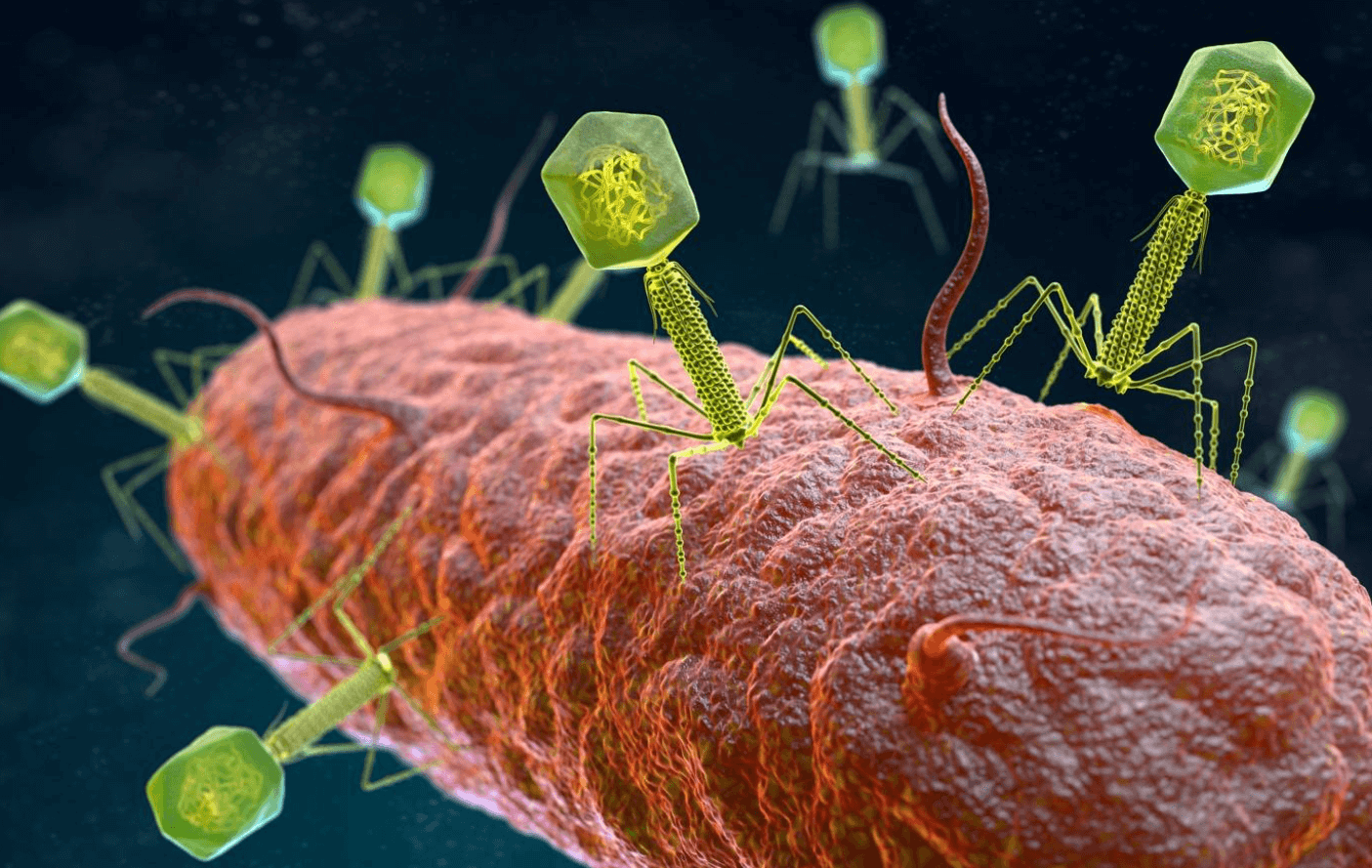
It’s also bit of a double win if successful with phage therapy offering us a potential avenue out of antibiotic reliance for dealing with bacterial infections. As a result, APT have partnered with the Antibacterial Resistance Leadership Group (ARLG) for the study with their CEO stating:
We are proud to be a part of this important trial and look forward to working with the ARLG and the CF community to bring new hope to those affected by this devastating disease and secondary respiratory infection.
Even more promisingly, this development does not stand alone. There has been a flurry of recent developments in the search for new CF treatments with the market for therapies predicted to become a multimillion-dollar industry in the next 5 years.
Gene therapies
The genetic nature of CF has made it a primary target for gene therapy approaches. However, gene therapy is still an infant technology when it comes to the clinical setting. The FDA has approved over 20,000 drugs in its 115-year history and only 5 gene therapies. That’s a ratio of over 4000:1. Doctor Anthony Davies, founder and CEO of founder of a gene therapy development and consultancy firm in California explained why:
There’s a massive pharma-economic problem once a blockbuster cell or gene therapy gets approved, such as for a solid tumor indication or one of the more common genetic diseases. They’re enormously more complex. They’re more expensive to manufacture and more complex to characterize.
Fortunately, the obstacles aren’t large enough to hinder all promising new developments in CF gene therapy. A recent study from the Yale CF Center reported the successful use of nanoparticles loaded with a gene therapy payload to correct CF-related mutations to the CFTR gene in mice.
“This is the first study to show that with a single intravenous administration of gene editing reagents multiple organs affected by CF can regain partial function of CFTR,” said Marie Egan, MD, director of the Yale CF Center.
The positive effect faded over time, but a treatment regime of repeated doses restored the therapeutic effect. The data represents the earlier stages of testing but is still a development that has the potential to have a huge benefit for CF patients
Despite advances like this, many still question if gene therapy is the best option; some have turned to other technologies. A partnership between Vertex and Moderna to create an mRNA based CF drug has already yielded a therapy that has cleared clinical trials and gained FDA approval and they aren’t finished there according to Moderna CEO Stéphane Bancel:
Moderna’s development of a proprietary inhalable lipid nanoparticle to deliver a functional cystic fibrosis treatment to the lungs could lead to a transformational medical achievement. We are excited by the progress that has been made with the upcoming advancement of VX-522 to the clinic and look forward to our ongoing collaboration to develop treatments for the underlying cause of cystic fibrosis.
Some children in France with CF recently started receiving an innovative treatment: a more ‘classic drug’ style formulation called Kaftrio, which increases the number of CFTR proteins on the cell surface and improves their activity and function.
So, this scattergun approach of looking at many types of therapy is bearing fruit, welcome news for CF patients across the globe.
The evolutionary reasons for high CF rates
The mutation that causes cystic fibrosis arose in the early Bronze Age and spread across Europe during ancient migrations. Cystic fibrosis is the most common fatal genetic disease in the US, and yet has genetic characteristics that should hinder it’s spread or remove the mutation from the gene pool altogether. We would expect natural selection to eliminate alleles with negative effects from a population, and yet many populations include individuals carrying such alleles. So why are these deleterious alleles still around? What might keep natural selection from getting rid of them?
First, up until recent advent of new therapies, the life expectancy of CF meant most patients died before the age of 14; years ago, most incidences weren’t diagnosed until victims were of parenthood age. The longer projected life expectancy means that survivors have more of an opportunity to pass along these killer mutations.
Second, CF is a recessive genetic disorder; this means both parents must carry one copy of the gene for a child to have a chance of being born with the disorder and, even then, the risk is one in 4. In most cases, these two characteristics would have limited the spread of the deadly gene.
However, in the case of CF, they created an evolutionary niche that has allowed the gene to grow in the population to the point where approximately 10 million Americans carry it. This is partly due to the gene’s recessive nature: you can carry one copy without exhibiting any symptoms of the disease. Only a genetic screening will alert you to the presence of the GF gene in your DNA. This has allowed the gene to ‘silently’ propagate though the population.
But still, the numbers of carriers don’t make sense. They are still too high. What else might be in play here?
The answer may lie in an unexpected place: the pandemics of respiratory illnesses that plagued generations in centuries gone by. It is proposed that carrying one copy of the gene for CF actually conferred an evolutionary benefit to prevent people from dying of tuberculosis and cholera. In other words, the negative effects of the genes involved were counterbalanced by their positive evolutionary contributions.
During the cholera epidemics of the 19th century that killed millions the primary cause of death was dehydration. A study in the early 1990s demonstrated that mice carrying one copy of the CF gene did not experience the same extent of diarrhea and dehydration and generally did not die when infected with cholera whereas ‘normal’ mice did. A more recent study suggested that CF patients themselves also had a higher resistance to cholera and, in a weird symbiosis, may have found symptomatic relief from CF if infected.

It’s all to do with the way the CFTR is involved in water transport in and out of cells. If you carried the gene for CF, your cells would hold onto water meaning you were less likely to succumb to dehydration and if you had CF, the thick mucus would prevent bacterial invasion creating much milder symptoms. In short, CF carriers and patients were more likely to survive — they had an evolutionary advantage.
The same also appears to be true for tuberculosis, which spreads by bacteria invading the lungs and creating an infection. And how do those bacteria get in? Through the very same CFTR channel that is dysfunctional in CF patients and partially dysfunctional in carriers. As a result, CF patients and carriers were once again protected against what was then a lethal disease that plagued communities across the globe.
It’s a fascinating theory, the tale of a mutation giving carriers a selective advantage against diseases that caused devastation to global populations. Carriers were more likely to survive and thus possessed an increased chance of passing the mutation down to the next generation. This hypothesis is now regarded by many as the best explanation for why such a lethal genetic disease became so common.
Sam Moxon has a PhD in tissue engineering and is currently a research fellow in the field of regenerative medicine. He is a freelance writer with an interest in the development of new technologies to enhance medical therapies. Follow him on Twitter @DrSamMoxon































