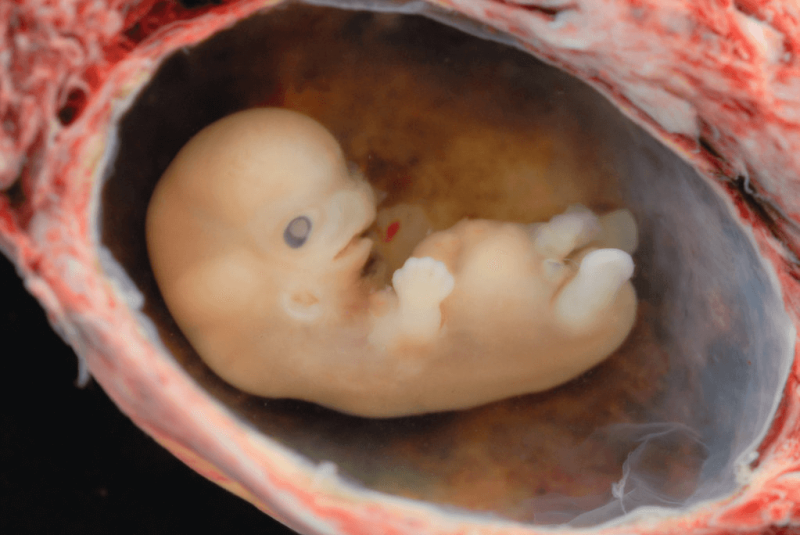
The early embryos that are being investigated are donated by women undergoing assisted reproductive technologies, or are nurtured from induced pluripotent stem cells, which are created by culturing skin cells (fibroblasts) in a brew of growth factors. The stem cells divide and differentiate into early embryos, but with only partial supportive structures, like the amnion and placenta, so development ceases before the fetal period begins at 8 weeks after fertilization. The stem cells provide a Goldilocks solution, glimpsing early embryos, but not sustaining their development past a few weeks.
Three new reports
In the June 20, 2023 issue of PLoS Biology, researchers from Germany, Spain, and the UK describe a newly-identified cell type that sets development on a clear path, then self-destructs, seemingly protecting the early embryo to which it clings. A woman undergoing IVF donated that embryo – it was an extra.
Women having abortions also donate embryos for research. A report in Nature from 2021 described the cell types in an aborted gastrula, two weeks post-fertilization. The gastrula, a critical stage when the three basic tissue layers appear as a body begins to take shape, revealed nerve cell and sperm precursors already being set aside. The woman donated through the Human Developmental Biology Resource, which grants automatic bioethical approval to donate embryos from the Institute of Human Genetics, Newcastle and the Institute of Child Health, London.
In two papers published [recently] in Nature, two research teams report growing embryo-like structures – embryoids – from stem cells, providing unprecedented glimpses into the critical time of implantation in the uterine lining. This is when many pregnancy losses, which may appear to be infertility, happen.
Identifying patterns of gene expression in the cells of an embryo reveals what the cells do, which is important because even cells that look identical under a microscope can differ greatly in their activities. An approach called single-cell transcriptomics is widely used to study gene expression by identifying the messenger RNAs (mRNAs) that a single cell produces.
The technique is called “RNA-Seq” for short. It reveals the biochemical goings-on as cells adhere into tissues, form sheets and blobs, undulate, fold, attach, and eventually sculpt organs, which are all present, in rudimentary form, 8 weeks after fertilization. (We biologists measure prenatal development from fertilization on – the 6-week “heartbeat bills” that limit abortion are actually, biologically speaking, 4 weeks. And “fertilization” is more precise, less emotional, than “conception.”)
Paradoxically, reproductive technology has sped ahead without us truly understanding the choreography of early embryogenesis. Despite the fact that more than 10 million people have been conceived with IVF, we still have a lot to learn. I’ve been thinking about this for a long time, since high school.
One of my first jobs was processing abortion remnants
In my senior year, I worked in a lab that processed the remnants of abortions. It was during the pre-Roe v. Wade spring of 1972 and pregnancy termination as a medical procedure was legal in New York. Thousands of women traveled there for that purpose – the lab was busy.
The bloody tissue arrived on a conveyer belt, in small, clear cups, like fruit cocktail. My job: slapping on labels. Being a budding biologist, I’d stare in fascination, swishing the contents, seeking tissues and bits of organs. But I had to keep up the pace – picture Lucy and Ethel on the assembly line in a chocolate factory in the famous episode of The Lucy Show.
For each little cup that went by, I didn’t have time to think about the heartache that likely led to the decision to end that pregnancy. Each one, I’m sure, had a story. But I wondered what could be learned from examining the contents, rather than discarding them.
A key developmental decision – Setting aside the inner cell mass
Six years after my lab job, when I was halfway towards getting my PhD in developmental genetics, Louise Joy Brown was born. The first “test tube baby” was conceived in glassware via in vitro fertilization. Today, IVF is so commonplace that extra fertilized ova and early embryos are routinely frozen, for future use. In some nations, researchers can easily access them to investigate the earliest steps of human prenatal development.
After fertilization comes a period called cleavage, when one cell divides into two, two into four, and so on until the embryo is a solid ball of cells (the morula), and then hollows out, becoming a blastula. Hugging its inner wall is a mere smear of cells designated the inner cell mass (icm). It grows and folds and contorts, forming a rudimentary three-layered embryo by the end of the second week. The shell of other cells, also derived from the original fertilized ovum (zygote), divides and specializes into supportive structures, essential for development to continue beyond a few weeks.
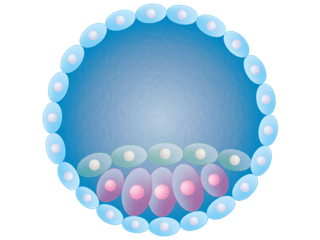
The new paper in PLoS Biology captures that critical time when cells “decide” whether to be part of the icm and the “embryo proper,” or remain in the outer circular shell, destined to develop as a supportive structure. The fleeting cell type that the researchers identified oversees that decision, perhaps unknown until now because of its ephemeral existence. But the elusive cell also displayed an unexpected oddity in utilizing its genome.
The newly discovered cell type in the embryo expresses jumping genes
Identifying a transient cell type that self-destructs when its job is done is exciting enough, but its actions are due to activating transposons – jumping genes.
About half of a human genome consists of DNA sequences that can move about the chromosomes. “New” transposons are those that we share with other primates only, meaning they jumped into our genomes relatively recently. “Old” transposons are more ancient, shared with more distantly related animals.
The researchers analyzed gene activity of each cell from 5-day old embryos and found that about a quarter of the cells didn’t have the expected mRNA signature of an early embryo or extra-embryonic membrane or pre-placenta. Instead, the unusual cells were packed with RNA versions of the “new” jumping genes. Enzymes can copy these RNAs back into DNA that inserts back into a chromosome, perhaps causing damage that could be the source of some pregnancy losses.
Later in development, the embryo cells harboring RNA copies of the new jumping genes divide, but give rise to cells with damaged DNA that then die. So the researchers compare the transcription of RNA copies of these jumping genes to a form of quality control that weeds out cells destined to doom the embryo.
“If a cell is damaged by the jumping genes, then the embryo is better off removing these cells. What we are seeing within embryos looks like survival of the fittest, between almost identical cells,” explained author Laurence Hurst, from the University of Bath. The team coined the term REject cells, in which RE stands for “retroelement” and “ject” for the fact that some cells are excluded from becoming part of an embryo.
Cells that become part of the inner cell mass, and therefore the embryo, not only don’t harbor RNA copies of the harmful jumping genes, but express a virus-like gene that helps suppress the young jumping genes. If this quality control process is too sensitive, the embryo may die, explaining some cases of apparent infertility that are really very early pregnancy losses.
Stem cells instead of embryos
A week after the PLoS Biology report on the REject cells, two papers in Nature describe using stem cells to grow tissues that model a human embryo from 9 to 14 days after fertilization. That includes another critical time in prenatal development, implantation into the uterine lining.
Berna Sozen and colleagues from Yale and the Max Planck Institute for Molecular Genetics in Berlin stimulated human pluripotent stem cells to divide, specialize, and organize into 3D structures that model the movements of an embryo as tissues form, layer, and intertwine, sculpting miniature body parts. RNA-Seq provided the molecular information to back up observations of cell types.
Their experiments follow the embryo-like structure – an embryoid – from fertilization, the initial rapid cell divisions (cleavage), then by day 6 formation of the inner cell mass, to implantation at day 7 and appearance of the three tissue layers by 24 days. A placenta never develops, so the embryoid ceases developing after a few weeks.
“Our system captures key features of human embryonic development … offering a reproducible, tractable, and scalable experimental platform to understand the basic cellular and molecular mechanisms that underlie human development, including new opportunities to dissect congenital pathologies with high throughput,” the researchers write. Unlike an embryo descended from a fertilized ovum, with the genomes of two individuals, an embryo derived from stem cells has both genome copies from one parent, the donor of the nucleus.
In the second paper in Nature… Magdalena Zernicka-Goetz and colleagues, in the US and UK, report using a different recipe to generate a mix of embryonic and extraembryonic parts, which intermingle and “self-organize” and “cross talk,” to “mimic several aspects of the post-implantation human embryo.” Adding a growth factor (bone morphogenetic protein) guides elaboration of the three-layered embryo, a bit of amnion, and even germ cell progenitors, which can go on to produce reproductive cells.
“We present a modular, tractable, integrated model of the human embryo that will allow us to probe key questions of human post-implantation development, a critical window when significant numbers of pregnancies fail,” the researchers write, stressing that “the model cannot implant and does not have the capacity to develop towards fetal stages.“
Coda: Will Right-to-Lifers comprehend the biology? Or misinterpret it?
I can’t help but wonder whether some individuals will object to these experiments, endowing skin-cell-derived doomed early embryos with the personhood status that is destroying women’s reproductive health care in an alarming number of US states. The utter lack of mention of anything biological in the Dobbs decision that revoked Roe v. Wade a little over a year ago suggests that some might indeed (I analyzed the entire case for its scientific and medical content at Genetic Literacy Project.)
Based on my reading of what wasn’t addressed in the Dobbs decision, I doubt that many folks waving anti-abortion signs know the distinction between a zygote and a pluripotent stem cell, an embryo and supportive structures, or even between an embryo and a fetus, and other nuances of developmental biology. Or RNA and DNA. But I hope I’m wrong, because pluripotent stem cells, their nuclei plucked from specialized “adult” cells, not embryos, can provide unprecedented views of the nuances of early prenatal development that may reveal how pregnancies go awry.
Ricki Lewis has a PhD in genetics and is a science writer and author of several human genetics books. She is an adjunct professor for the Alden March Bioethics Institute at Albany Medical College. Follow her at her website or X @rickilewis
A version of this article was originally posted at PLOS and has been reposted here with permission. Any reposting should credit the original author and provide links to both the GLP and the original article. Find PLOS on X @PLOS