The hottest controversy today in human genetics research is oocyte modification. Earlier this year, the Food and Drug Administration concluded an initial set of hearings on the proposed procedure.
The technology is technically known as assisted reproduction for the prevention of transmission of mitochondrial disease or treatment of infertility. Scientists achieve this by replacing mitochondrial DNA (from one genetic mother) with mtDNA from another mother to avert the potential to have offspring with certain genetic mutations.
The mutations targeted, because of the oocyte modification technology, would be only mitochondrial DNA-related (mtDNA). These mutations, however, are particularly pernicious because mitochondrial DNA are thought to have a high mis-code rate and copy very quickly in relative terms.
The jury is still out on some of this, because estimates for the mutation rate in mtDNA can often vary by two orders of magnitude and are based on historical analysis where error variance is high. This work on oocyte modification has already demonstrated efficacy in vitro, so the next major step would be human clinical trials.That’s why the FDA panel was held. The hearings brought out some die-hard opponents of human genetic engineering.
Marcy Darnovsky, executive director of the Berkeley, California-based Center for Genetics and Society, has stated that pursuing such technology needs to be done cautiously, and that “Life is full of slippery slopes and we need brakes.”
The FDA panel discussion did not intend to address the ethics of the technology, which is really likely to be where the public might voice concerns. Instead, the FDA panel focused on the objective scientific realities of this technology, including future stability of the mtDNA, whether the procedure would be a long term solution for certain congenital diseases, and the safety of carryover of mitochondrial DNA to successive generations of offspring.
Similar considerations are also taking place in the United Kingdom, spearheaded by the Nuffield Council on Bioethics in London, an independent foundation. Although Nuffield has not been as direct and open as the FDA about the scientific rigor of the procedure, it has more directly addressed ethical considerations. The council members have suggested that if the technology has adequate safety to progress successfully through, for example, Britain’s MHRA (Medicines and Healthcare products Regulatory Agency), EMA (European Medicines Agency), and/or FDA screening, that it would automatically be ethical for families to use to block heritable mitochondrial DNA-associated disease.
The Nuffield Council’s current stance: “Due to the health and social benefits to individuals and families of living free from mitochondrial disorders, and where potential parents express a preference to have genetically-related children, on balance we believe that if these novel techniques are adequately proven to be acceptably safe and effective as treatments, it would be ethical for families to use them.”
There is a very real potential that the build-up of mtDNA mutations over a woman’s lifespan might be linked to a decline in female fertility. Because of this hypothetical mechanism, this oocyte modification technology could increase age-advanced fertility rates. This has, so far, only been explored in the case of female oocyte agenesis (lack of egg production) and not in cases of lower infertility in previously fertile women or other age-related declines in fertility.
As one might expect, there is likely a broad range of opinions about such a contentious issue, ranging from ‘why not do everything possible’ at one tail of the distribution curve to ‘we shouldn’t be tampering with this’ at the other end. It could be presumed that most of the public will fall in the middle of the distribution curve, as is typically the case (Fig 1). But it’s entirely possible that this technology could represent an unparalleled polarization of the public in terms of very few moderate responses, and a preponderance of extreme reactions (Fig 2).
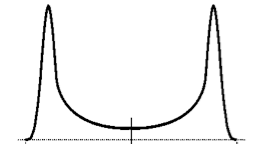
We refer to such an extreme case as a “bi-modal” distribution, and when applied to ethico-politics, these are issues that are very difficult to find a middle ground. As the genetics revolution advances, we may find more-and-more of these bi-modal ethical controversies– a vast gulf of disagreement in perceptions. On one side there will be those who want to explore what is possible and use the Scientific Method as their arbiter of what is and what isn’t; and on the other side will be those who choose to be guided by intuition and anecdote.
Ben Locwin, PhD, MBA is a contributor to the Genetic Literacy Project and is an author of a wide variety of scientific articles for books and magazines. He is also a researcher and consultant for a variety of industries including behavioral and psychological, food and nutrition, and pharmaceutical. Follow him on Twitter @BenLocwin.































