Is there a gene-based magic elixir that could help treat male infertility?
For the first time, scientists have found a way to coax an embryonic stem cell to become a sperm cell, the Chinese researchers also reported the sperm cells were capable of fertilizing an egg and producing a viable offspring.
The study could have major implications for treating male infertility in humans, but some scientists are speaking cautiously about the technique noting that there are still several biological and ethical obstacles to overcome before this achievement can make an impact in the clinic.
The study built on previous work by Takashi Shinohara, a reproductive biologist at Kyoto University in Japan, who in 2011 led a team that was the first to turn embryonic stem cells into primordial germ cells (PGCs) in vitro. These are specialized cells that, under specific conditions, can become an egg or a sperm cell. This latest study was able to do more of the process in a culture dish.
The team was led by Xiao-Yang Zhao, developmental biologist at the Southern Medical University in Guangzhou, and Qi Zhou, a cloning specialist and stem-cell biologist at the Institute of Zoology in Beijing and the results were published in Cell Stem Cell. They began by making the PGCs and then exposing these cells in a culture dish to tissue from a mice testes. This tissue contained cells that are responsible for providing the PGCs with nutrients and the growth signals that initiate the process of meiosis — which cuts a cell’s genome in half (i.e. one set of chromosomes). Sperm cells need to have half of a genome so that when it combines with an egg (which also has half a genome) the resulting fertilized embryo will have a complete genome.
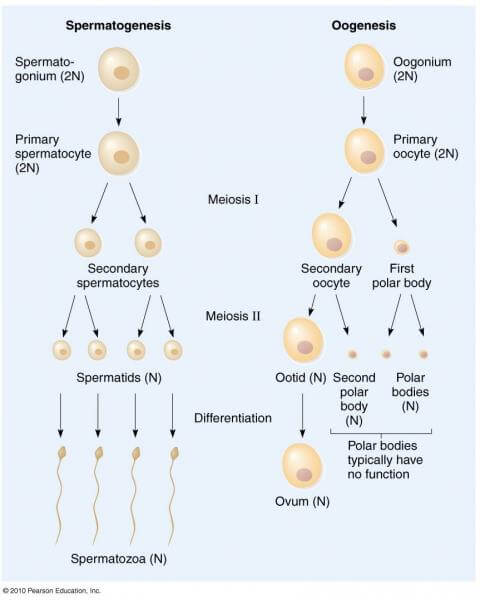
PGCs have a full genome, but when Zhao and Zhou’s team grew these PGCSs in the presence of testes tissues in a culture dish after 14 days, the PGCs underwent meiosis to be become cells that resembled spermatid cells. These are cells that have half a genome, but still lack some distinguishing features of a mature sperm cell, most notably a tail. But because it has the right chromosome number, the scientists were able to inject these spermatid cells directly into an egg and fertilization occurred. The scientists reported that 15 months later, the mice created from this fertilization were healthy and able to give natural birth.
The results have been received with excitement in the fields of reproductive technology, according to researchers who commented to GLP sister site the Genetic Expert News Service, which gathered expert reactions to the study.
Kyle Orwig, a professor of obstetrics, gynecology, and reproductive Sciences, at the University of Pittsburgh School of Medicinet:
This is an important achievement. The important thing is that they have demonstrated the concept in mice, validated by the production of viable offspring. Wow! Only time will tell if this method will work in human cells. Several excellent labs have tried to achieve the result described in this study, most with limited success. In that regard, this is a landmark report. However, as with all important scientific advances, it is essential that the results be replicated and confirmed in other laboratories.
Vittorio Sebastiano, assistant professor in the department of obstetrics and gynecology at Stanford University School of Medicine, who is doing similar research to Zhao and Zhou, noted the important historical context of the research:
This study is certainly a milestone in the field. While in vitro derivation of germ cells capable of meiosis had already been proven to some extent, the work of this group surpasses previous attempts, especially considering that the cells were capable of giving rise to viable embryos that could develop into mouse pups. This “functional” test represents the gold standard and until now no-one had been able to show this using an in vitro-only approach.
Despite widespread excitement, some scientists point to potential pitfalls in the research. Sebastiano and Orwig each noted that biologically, the technique is not directly translatable to humans. Zhao and Zhou used embryonic stem cells (ESCs), which are uniquely biologically malleability, but an adult human male donor will not have these cells to donate to be used to make new sperm cells. Orwig noted, “the fact that the ESCs would not be obtained from the person being treated, so would not enable him to have a genetically related child.”
But both researchers believed the technique could apply to humans if ESCs were replaced with induced pluripotent stem cells (iPSCs). These are cells from an adult that are ‘reprogrammed’ to possess a similar malleability to embryonic stem cells. But Sebastiano believed that would raise a whole host of other ethical dilemmas:
Although this approach is not in principle different from being able to generate any other “dysfunctional” organ from patient-derived iPSCs, the fact that we are not simply restoring the functionality but potentially creating cells that could be used to generate embryos raises an endless number of ethical questions and concerns that we have not yet fully discussed. The reason is very simple: whatever alteration we may cause during the process of reprogramming or differentiation of the cells will be passed on to the following generations, and we cannot predict how this could impact the life of the babies that would be conceived using this technique.
Peter Donovan, professor at the Sue and Bill Gross Stem Cell Research Center at the University of California, Irvine, pointed to other shortcomings. Donovan noted that some data suggest that genes for PGC development in humans are very different from those in mice, which will could slow the translation of this technique to humans. He said the study also raised several ethical questions:
…How can we tell if spermatids made in a laboratory are really of the same high quality as those made and tested by natural selection in the testis? How do we know that spermatids made in the lab have not acquired mutations to DNA? Science may well answer these questions in time.
The ethical and biological hurdles will probably keep this technique out of fertility clinics for some time, but Terry Hassold, professor at the school of molecular biosciences at Washington State University, said that doesn’t mean the technique won’t eventually get there:
There’s no question that the technique needs to be improved further, but if we can build on this as a technique that is doable in the laboratory, it really is going to change the way we think about cases of infertility that are currently unable to be treated by IVF. The crucial question is whether someone else going to be able to replicate the study, and if that is the case then it really will revolutionize assisted reproduction as we know it. All the IVF clinics would be likely to hop on it, although in my opinion that would be very premature.
Nicholas Staropoli is the associate director of GLP and director of the Epigenetics Literacy Project. He has an M.A. in biology from DePaul University and a B.S. in biomedical sciences from Marist College. Follow him on twitter @NickfrmBoston.