That could block the coronavirus in the nose, before it can travel down to the lungs or be coughed onto another person, perhaps becoming a powerful partner to vaccines and therapeutics, and easy to administer, store, and ship.
It “could allow us to reduce transmission and be able to have a quick response to outbreaks in certain areas of the world,” said Jeffrey Nau, CEO of Oyster Point Pharma, based in Princeton NJ. The company recently announced repurposing of the smoking-cessation pill Chantix™ (varenacline), as well as a second molecule in the same class, simpinicline, each as nasal sprays against COVID. The FDA approved Chantix, a Pfizer product, in 2006. Today nearly 400 clinical trials are exploring other uses.

Oyster Point had been developing the drug in nasal spray form to treat dry eye disease when the pandemic began, announcing a phase 3 clinical trial in May. Establishing safety for dry eyes may shorten the regulatory road for the COVID application, said Nau. Experiments in monkeys and human cells show that the nasal spray inhibits viral replication within 24 hours of administration to the nasal cavity.
The nasal spray might straddle the time period of infection, useful for “pre-exposure prophylaxis,” lowering risk before encountering an infected individual (like PrEP to prevent HIV/AIDS), as well as for “post-exposure prophylaxis,” after encountering someone who tests positive for COVID.
With the delta variant easily flitting from person to person, even among the vaccinated, an early-acting nasal spray is of potential enormous value. And the part of the virus that the drug targets is relatively common to all variants identified so far.
The rerouting of a dry eye drug to a weapon against SARS-CoV-2 arose from a fascinating convergence of observations. The story brings to mind such dictums as “thinking outside the box,” “expect the unexpected,” and “chance favors the prepared mind.”
Retooling a doorway into cells lining the nose
The anatomical connection between the eyes and the nose lies behind the mechanism of how a dry eye drug also works against a respiratory virus. Both the nasal passages and the cornea are festooned with the ACE2 receptors to which the virus readily binds.
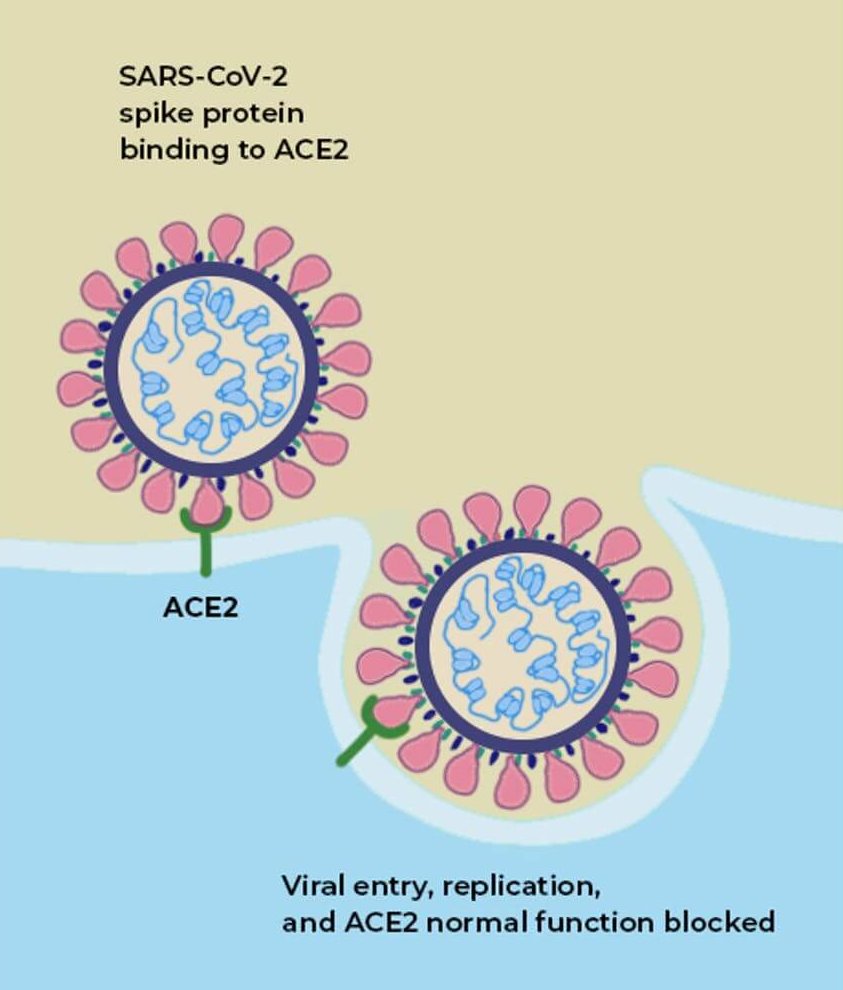
SARS-CoV-2 infects cells lining the inside of the nose, but it also drains through the sinuses from an infected cornea, all the while replicating like crazy. Viral replication can begin before a vaccine’s protection kicks in, which is why and how vaccinated individuals can continue to spread the virus, until (and if) we reach herd immunity.
“We think of the cornerstone of dealing with the pandemic through vaccines, but we need other tools in the arsenal to stop transmission and provide pre- and post- exposure prophylaxis. The eye-nose connection is really important for that,” said Nau.
The eye drugs work based on receptor biology. Receptors are proteins embedded in cell membranes to which certain molecules can bind. The binding either activates a receptor, producing a biological response, or blocks the receptor and the response. An activator is termed an agonist; a blocker, an antagonist.
The dry eye drugs are agonists, turning on nicotinic acetylcholine (nACh) receptors on the nerve cells in the nose that activate and maintain the production of natural tear film. Such a receptor consists of 5 parts surrounding an opening, resembling a flower with 5 petals. In the body the neurotransmitter acetylcholine binds these receptors, triggering various nerves to stimulate muscles to contract, activate other nerves, and release chemicals in the brain. But nicotine fits the receptor too – like a catcher’s mitt that can hold both a softball and a hardball.
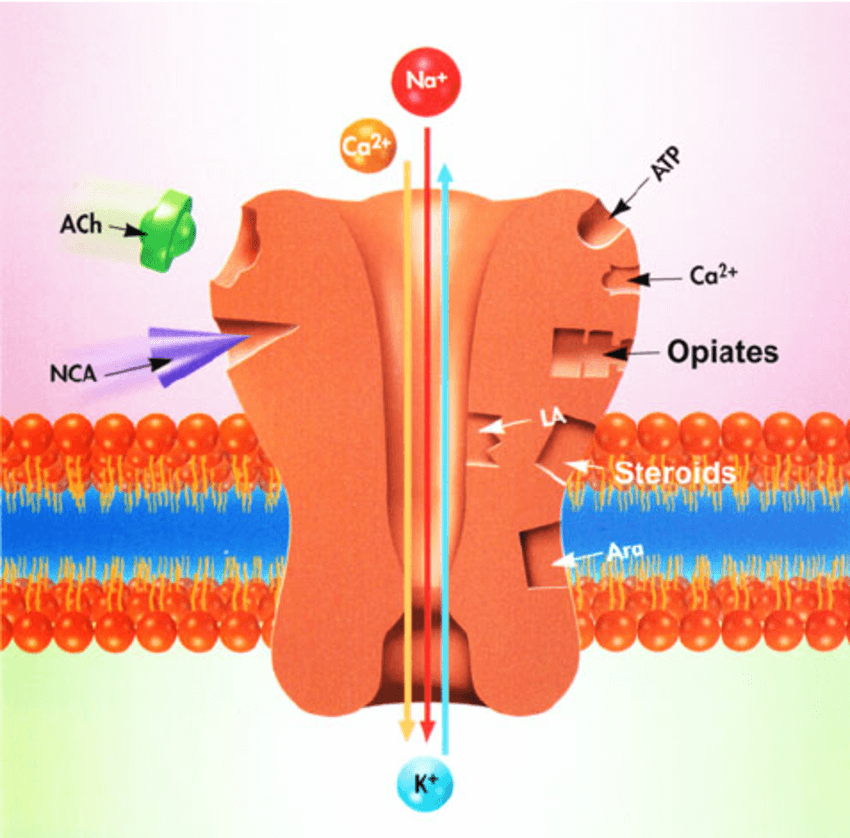
The binding of the different molecules to nACh receptors lies behind the reasoning to repurpose the dry eye drugs to block COVID:
- Smoking may be a strong risk factor for severe COVID because nicotine strongly binds the nACh receptors and may interfere with viral attachment to ACE2 receptors.
- A loop in the spike protein of SARS-CoV-2 grabs onto nACh receptors.
- A tiny part of the spike protein resembles the location where snake venom toxins bind to nACh receptors – and snake venom paralyzes victims by binding these receptors on muscle cells.
Other clues to the role of nACh receptors in COVID come from symptoms. The senses of taste and smell, which are altered or absent in some patients, may arise from viruses binding to nACh receptors in the brain’s smell center and on taste cells of the tongue. In fact, nicotine overdose, which can affect people harvesting tobacco, causes headache, dizziness and weakness similar to what happens in COVID. The receptors also dot cells in the stomach and intestines where the gastrointestinal distress of COVID arises. Another clue that points to the virus interacting with these receptors comes from COVID patients who produce auto-antibodies that bind and turn off the receptors at muscle cell junctions, causing an autoimmune condition similar to the muscle weakness of myasthenia gravis.
The rationale behind redirecting a dry eye drug against COVID
If SARS-CoV-2 and varenacline compete for the same receptors, might the dry eye drug that came from the smoking-cessation drug also block the virus from glomming onto our cells? To find out, researchers at Oyster Point collaborated with colleagues at the Trudeau Institute in Saranac Lake, NY for a series of preclinical experiments. “The nicotinic acetylcholine receptor system interaction with the SARS-CoV-2 infection pathway is not well understood and could potentially bring additional therapeutics to the forefront,” said Nau.
The researchers found that varenicline binds a stretch of 11 amino acids in the spike protein, which prevents the virus from binding the nACh receptors. And because this class of drugs seems so far to block all viral variants, the drugs may work on almost any version of the pathogen. Varenicline clearly keeps out the original wild type virus, the alpha (UK) variant, and the beta (South African) variant, in human lung and intestinal cells growing in glassware, and the company is testing the drug against the delta and lambda variants. A preprint describing the experiments is here.
Even more encouraging were results of experiments in rhesus macaques. On the first day of the studies, the monkeys received snorts of varenicline before and after infection with a whopping dose of the virus. Several more treatments over the next 6 days were in tune to the natural course of infection in the species. The researchers collected nasal swabs to test for virus on days 1, 2, 4, and 5, and then the animals were euthanized and their tissues examined.
The nose swabs revealed an enormous reduction in viral genomes in sync to the treatments. But what really excited Nau was the reduction in specifically the parts of the genome that encode the spike and envelope proteins, which are key to establishing infection:
Compared to control animals, by day 3 we were not able to assay subgenomic RNA in nasal swabs because it is below the level of quantification. This may mean that we can potentially reduce transmission and infectivity of the virus.
Coda
It is difficult, impossible even, to look ahead to the path that the novel coronavirus will take. But it’s now clear that global distribution of vaccines to wipe the virus off the Earth – the smallpox model – likely will never be achieved for SARS-CoV-2 due to logistical roadblocks and the unprecedented refusal of those who have access to vaccines to receive them, making herd immunity impossible. Nor can we expect the virus to just go away more or less on its own, as its predecessor SARS did by 2004.
Diverse treatments are going to be critical to control a virus that might linger among us, surging periodically when public health measures lapse, for many years to come. It’s easy to envision an antiviral nasal spray making a major contribution to controlling COVID, on a global level… if it works.
Ricki Lewis has a PhD in genetics and is a science writer and author of several human genetics books. She is an adjunct professor for the Alden March Bioethics Institute at Albany Medical College. Follow her at her website or Twitter @rickilewis































