Indeed, the Biosafety Protocol (Cartagena agreement) and its Nagoya – Kuala Lumpur Supplementary Protocol on Liability and Redress have been built on that unscientific mistake; beyond its anti-biotech stance, that treaty may use its “anti-GMO” keystone as a mask for protectionist intents. Moreover, oppressive and arbitrary red tape goes against the aims of the Convention on Biological Diversity, of which the Biosafety Protocol is a contradictory offspring. Almost twenty years after the entering into force of the Protocol, taking stock of its poor results means realizing that there is an ongoing waste of resources to implement its utterly wrongheaded rules. Even worse, nobody can calculate the opportunity costs, i.e. the lost occasions of biodiversity conservation, agricultural progress and balanced development which have been impeded, mostly in low-income countries.
The misguided and counterproductive treaty is heavily detrimental. A serious reflection about its desirable abolishment and a better utilization of financial means and human capabilities for agricultural progress, above all in the developing world, is badly needed.
Introduction
The ongoing development of “green” biotechnologies raises questions, problems and the need for regulation in three broad areas: the healthiness of agricultural products, i.e. food and feed produce; the impact on natural environments, which are more or less affected by the interventions connected to cultivation and animal husbandry; and the complex social, legal, ethical, political and economic issues involved. On the environmental subjects, the primary international organization is the Convention on Biological Diversity of the United Nations Environment Program (Convention on Biological Diversity 1992). The Convention is an international treaty, the aim of which is to reconcile the reasonable use of technologies, including agricultural technologies, with the highest possible protection of the environment and the diversity of nature.

Within this wide area of scientific, legal and juridical issues, particular attention has been attracted, in recent decades, by so-called “Genetically Modified Organisms”: but this expression and the oft cited acronym “GMOs” (inverted commas are mandatory, due to the unsteadiness of its supposed grounds), used with reference to an ill-defined bunch of agricultural products (mostly crops), obtained via several different recombinant DNA (rDNA) techniques, does not indicate a consistent group of objects, with even a minimal amount of homogeneity. For many years a rickety fence has been erected on a fuzzy border, which is supposed to separate “GMOs” from other biotech methods (comprising physical and chemical mutagenesis), even when the traits obtained are the same (e.g. resistance to pests, tolerance to herbicides, reduced risks of toxicity or allergenicity, higher levels of nutrients, ability to thrive in adverse conditions such as drought, flood and climate change).
Since the early 1980s, life scientists have been recommending any regulatory approach to be focused on single (agricultural) products, not on the processes (Tagliabue 2017) used to create new varieties of plants or microorganisms or animals. Therefore, most experts think (e.g. Ammann 2014) that the supposed “GMO” ensemble is actually void of any taxonomic or even semantic value: many scientific societies (National Research Council 2004; American Society for Microbiology 2000; American Phytopathological Society 2001; American Society of Plant Biologists 2006; American Society for Cell Biology 2009) have repeatedly stated that the pseudo-concept has no basis in biology and genetics, let alone in agronomy; numerous scientists affirmed the same stance. (Prakash et al. 2000-2014. Statement signed, since 2000, by 3400 scientists, among them 25 Nobel laureates)
In other words, there is no theoretical basis to establish an a priori distinction among various agri-food groups of biotechniques, as far as potential risks for human/animal/plant or the environment are concerned; from an epistemological point of view, even the outcomes of natural variations are relatively unpredictable: “conjectural risks of genetic engineering must be of the same order as those for natural biological evolution and for conventional breeding methods. […] There is no scientific reason to assume special long-term risks for GM crops.” (Arber 2010, Abstract)
Thus, single new agri-food cultivars, deriving from any method, on a case by case approach, should undergo rigorous tests and exams, which have to be proportional to the potential risk (e.g. new potato varieties are usually checked for solanine level); on the contrary, diverse “GMOs” cannot be subject, as a supposed ensemble, to all-encompassing evaluations, whether positive or negative. Note that if other groups of products, e.g. those 3000+ varieties obtained via mutagenesis (FAO-IAEA 2017) were singled out to be governed apart for the sole reason that they derive from certain processes, the rationale would be equally anti-scientific: yet, only “GMOs”, in most regulators’ eyes, form a caste that merits unwarranted suspicion.
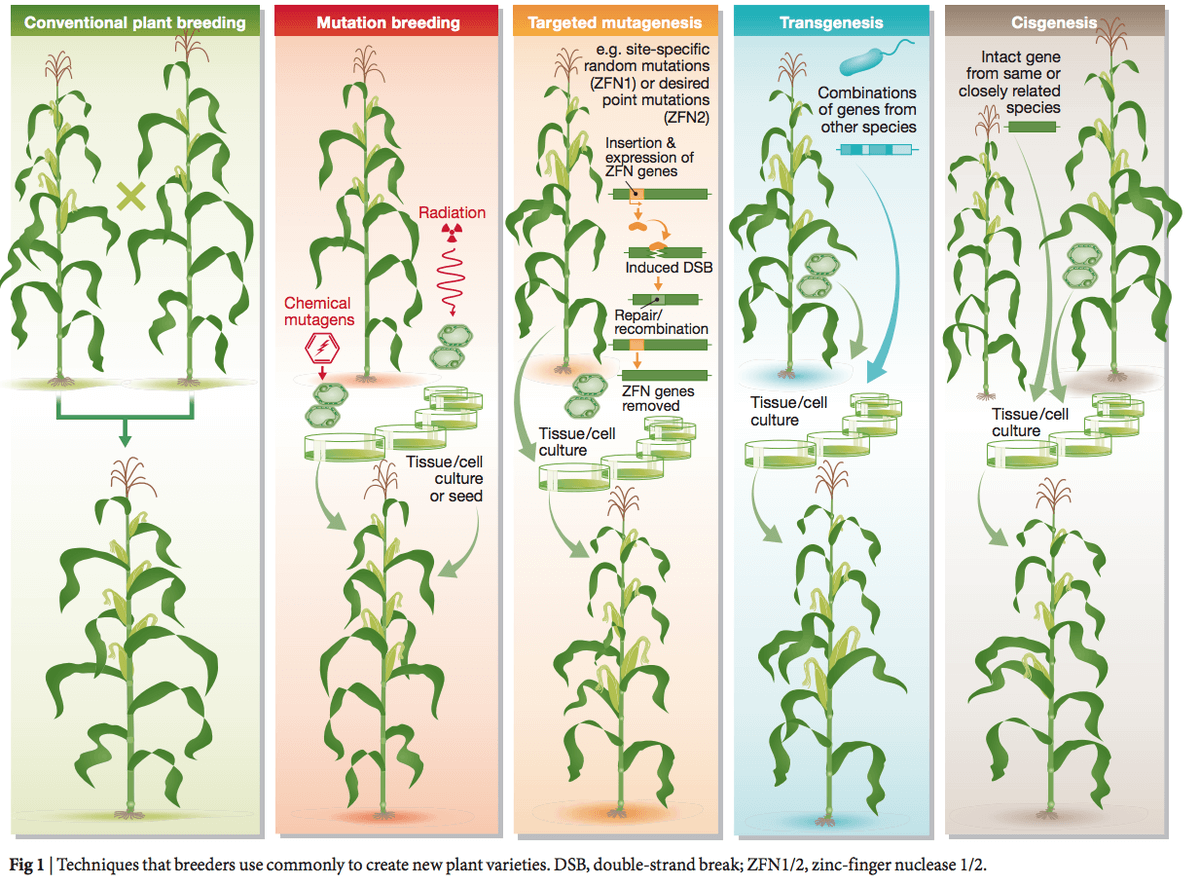
To be clear, the confirmed safety and healthiness of each currently commercialized product from different biotechnologies do not represent any guarantee that a negative impact on the environment or health cannot appear in future products (rDNA or otherwise; agricultural-”green” or otherwise: e.g. “red”, pharmaceutical or “white”, industrial). The outcomes of more or less traditional biotech applications frequently do not live up to expectations: it happens all the time, the results of failed experiments ending up in the waste bin. The meaningless distinction between recombinant DNA cultivars and other similar agri-food products should be replaced by a meaningful divide between healthy, well-controlled foods/feeds and problematic or unsatisfactory ones – which are ditched.
Therefore, according to the scientific consensus (Tagliabue 2016b), recombinant DNA crops as a supposed entity cannot form the basis of any biotech sectoral regulation. Notwithstanding that, lawmakers at national and regional (European Union) levels have created specific rules to be applied to “GMOs”, and such an approach has also reverberated at international level. Our interest is in particular in an annex to the Convention on Biological Diversity, the Cartagena Protocol on Biosafety, with its Nagoya – Kuala Lumpur Supplementary Protocol on Liability and Redress.
Convention on Biological Diversity and Biotechnologies: Promises and suspicions
At the first United Nations Conference on Environment and Development (UNCED), which took place in Rio de Janeiro in 1992, there was the possibility of all the nations signing a very important international treaty, the Convention on Biological Diversity (Convention on Biological Diversity 1992) – known informally as the Biodiversity Convention – which seeks to safeguard biodiversity, the long-term use of its elements and the fair division of the advantages from the exploitation of genetic resources. The Convention is legally binding: the signatory states are required to implement its contents; many nations around the globe have ratified it, with the important exception of crucial states such as the USA, Russia and Australia. (https://bch.cbd.int/protocol/parties. Last update 3 May 2018)
Let us look at how biotechnologies in general, and “GMOs” in particular, are regulated in the text of the treaty. There are three points which touch on our discussion.
In Art. 8 of the Convention, dedicated to “In-situ conservation”, at point (g), the contracting parties agree to “Establish or maintain means to regulate, manage or control the risks associated with the use and release of living modified organisms resulting from biotechnology which are likely to have adverse environmental impacts that could affect the conservation and sustainable use of biological diversity, taking also into account the risks to human health”.
“Article 16. Access to and Transfer of Technology 1. Each Contracting Party, recognizing that technology includes biotechnology, and that both access to and transfer of technology among Contracting Parties are essential elements for the attainment of the objectives of this Convention, undertakes […] to provide and/or facilitate access for and transfer to other Contracting Parties of technologies that are relevant to the conservation and sustainable use of biological diversity or make use of genetic resources and do not cause significant damage to the environment.”
“Article 19. Handling of Biotechnology and Distribution of its Benefits […] 3. The Parties shall consider the need for and modalities of a protocol setting out appropriate procedures, including, in particular, advance informed agreement, in the field of the safe transfer, handling and use of any living modified organism resulting from biotechnology that may have adverse effect on the conservation and sustainable use of biological diversity.”
The second of the above citations thus expresses confidence in the role of technology in general, and biotechnologies in particular, committing signatories to use and disseminate them, if and to the extent they favour the safeguarding of biodiversity and do not excessively harm the environment; instead, the first citation expresses a suspicion about the “risks” which “living modified organisms” (this is the specific expression chosen by the Convention to indicate “GMOs”, and we will later see why) may have on the environment and health: for this, the third citation augurs the drafting of an ad hoc protocol to manage such particular organisms, imagining that they may have negative effects on biodiversity. The theoretical picture seems therefore clear: confidence asserted in biotechnologies is combined with a general fear that rDNA organisms “may have” negative consequences. The protocol to manage the dreaded risks was then drawn up some years later: this is the document which we are interested in looking closely at.
Notes:
(1) The Introduction is not part of the text of the Protocol but appears in the official booklet published in English.
(2) Various experts comment on the article; even when they do not openly support dumping of the Protocol, they highlight its inadequacy: www.scidev.net/global/policy/opinion/the-cartagena-protocol-the-debate-goes-on.html (accessed 30 October 2019).
(3) This must not be confused with the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (Convention on Biological Diversity 2010c), a treaty which was also approved in 2010 in the same location and is directly connected to the Convention on Biodiversity.
(4) “We continue to lose biodiversity at a rate never before seen in history – extinction rates may be up to 1,000 times higher than the historical background rate.” (Convention on Biological Diversity 2010a, Message from the Executive Secretary, p. 2)
References:
Adler, Jonathan H. 2000a. More Sorry than Safe: Assessing the Precautionary Principle and the Proposed International Biosafety Protocol. Texas International Law Journal 35:173-205. http://scholarlycommons.law.case.edu/faculty_publications/226
Adler Jonathan H. 2000b. The Cartagena Protocol and Biological Diversity: Biosafe or Biosorry? Georgetown International Environmental Law Review 12:761-777. http://scholarlycommons.law.case.edu/faculty_publications/190
Allen, Kate. 2013. Meet Calestous Juma, Africa’s genetically modified crop ‘optimist’. Toronto Star, June 17. www.thestar.com/news/world/2013/06/17/meet_calestous_juma_africas_genetically_modified_crop_optimist.html
American Phytopathological Society. 2001. Biotechnology and Its Application to Plant Pathology.
www.apsnet.org/members/outreach/ppb/positionstatements/Pages/Biotechnology.aspx
American Society for Cell Biology. 2009. Statement in Support of Research on Genetically Modified Organisms. www.ascb.org/index.php?option=com_content&view=article&id=315&Itemid=31
American Society for Microbiology. 2000. Statement on Genetically Modified Organisms. www.asm.org/index.php?option=com_content&view=article&id=3656&Itemid=341
American Society of Plant Biologists. 2006. Statement on Plant Genetic Engineering.
Ammann, Klaus. 2014. Genomic Misconception: A fresh look at the biosafety of transgenic and conventional crops. A plea for a process agnostic regulation. New Biotechnology 31:1-17.
Arber, Werner. 2010. Genetic engineering compared to natural genetic variations. New Biotechnology 27:517-521.
BASF (2018) Clearfield® Production Systems. https://agriculture.basf.com/us/en/Crop-Protection/Clearfield.html
Berg, Paul. 2008. Meetings that changed the world: Asilomar 1975: DNA modification secured. Nature 455:290-291.
Bernauer, Thomas, and Philipp Aerni. 2008. Trade Conflict Over Genetically Modified Organisms. Pp. 183-193 in Handbook on Trade and the Environment, edited by Kevin P. Gallagher. Cheltenham: Edward Elgar.
Buechle, Kurt. 2001. The Great Global Promise of Genetically Modified Organisms: Overcoming Fear, Misconceptions, and the Cartagena Protocol on Biosafety. Indiana Journal of Global Legal Studies 9:283-324.
Cantley, Mark. 1995. The Regulation of Modern Biotechnology: A Historical and European Perspective: A Case Study in How Societies Cope with New Knowledge in the Last Quarter of the Twentieth Century. Pp. 508-681 in Biotechnology, edited by H.-J. Rehm and G. Reed. Weinheim: Wiley-VCH Verlag.
Colorado State University, Department of Soil and Crop Sciences. 2004. Discontinued Transgenic Products. http://cls.casa.colostate.edu/transgeniccrops/defunct.html
Convention on Biological Diversity. 1992. Convention on Biological Diversity (text of the treaty), Rio de Janeiro: United Nations, 5 June 1992. www.cbd.int/convention/text
Convention on Biological Diversity. 2000. Cartagena Protocol on Biosafety to the Convention on Biological Diversity: text and annexes, Montreal: Secretariat of the Convention on Biological Diversity, 2000. http://bch.cbd.int/database/attachment/?id=10694
Convention on Biological Diversity. 2010a. Global Biodiversity Outlook 3 – Executive Summary. www.cbd.int/gbo3
Convention on Biological Diversity. 2010b. Nagoya – Kuala Lumpur Supplementary Protocol on Liability and Redress. http://bch.cbd.int/protocol/NKL_text.shtml
Convention on Biological Diversity. 2010c. Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization. www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf
Dubock, Adrian. 2014. The politics of Golden Rice. GM Crops & Food 5:210-222.
FAO-IAEA – Food and Agriculture Organization of the United Nations, International Atomic Energy Agency. 2017. Mutant Variety and Genetic Stock (MVGS) Database, http://mvgs.iaea.org
Guruswamy, Lakshman D. 2002. Sustainable Agriculture: Do GMOs Imperil Biosafety? Indiana Journal of Global Legal Studies 9:461-500. www.repository.law.indiana.edu/cgi/viewcontent.cgi?article=1243&context=ijgls
Herring, Ronald J. 2010. Framing the GMO: Epistemic Brokers, Authoritative Knowledge, and Diffusion of Opposition to Biotechnology. Pp. 78-96 in The Diffusion of Social Movements: Actors, Mechanisms, and Political Effects, edited by Kolins Givan, Rebecca, Kenneth M. Roberts, and Sarah H. Soule. Cambridge, Mass.: Cambridge University Press.
Hobbs, Anna L., Jill E. Hobbs, and William A. Kerr. 2005. The Biosafety Protocol: Multilateral Agreement on Protecting the Environment or Protectionist Club? Journal of World Trade 39:281-300.
Hurtado, María Elena. 2013. Ten years in: Taking stock of the biosafety protocol. SciDevNet (website), 28 September. www.scidev.net/global/gm/feature/ten-years-in-taking-stock-of-the-biosafety-protocol.html
ISAAA – International Service for the Acquisition of Agri-biotech Applications. 2019. Brief 54: Global Status of Commercialized Biotech/GM Crops: 2018. Ithaca (NY): ISAAA. www.isaaa.org/resources/publications/briefs/54/
IUCN – International Union for the Conservation of Nature. 2007. Current Knowledge of the Impacts of Genetically Modified Organisms on Biodiversity and Human Health. http://cmsdata.iucn.org/downloads/ip_gmo_09_2007_1_.pdf
Juma, Calestous. 2013. Persecuting Biotechnology. Technology and Policy (website), 11 January 2013. www.belfercenter.org/publication/persecuting-biotechnology
Kerr, William A., Stuart Smyth, Peter W.B. Phillips, and Martin Phillipson. 2014. Conflicting Rules for the International Trade of GM Products: Does International Law Provide a Solution? AgBioForum 17:2. www.agbioforum.org/v17n2/v17n2a02-kerr.htm
Kinderlerer, Julian. 2008. The Cartagena Protocol on Biosafety. Collection of Biosafety Reviews 4:12-65. www.icgeb.org/~bsafesrv/pdffiles/Kinderlerer.pdf
Komen, John. 2012. The emerging international regulatory framework for biotechnology. GM Crops and Food 3:78-84.
National Research Council. 2004. Safety of Genetically Engineered Foods. Washington, DC: National Academy Press.
Nature Biotechnology. 2000. Swapping science for consensus in Montreal (Opinion – editorial). Nature Biotechnology 18:239.
Nicolia, Alessandro, Alberto Manzo, Fabio Veronesi, and Daniele Rosellini. 2014. An overview of the last 10 years of genetically engineered crop safety research. Critical Reviews in Biotechnology 34:77-88.
OECD – Organisation For Economic Co-Operation And Development. 1986. Recombinant DNA safety considerations. Paris: OECD.
Prakash, Channapatna S. et al. 2000-2014. Scientists In Support Of Agricultural Biotechnology (declaration and petition). www.agbioworld.org/declaration/petition/petition.php
Shibata, Akiho. 2013. Introduction. Pp. 1-14 in International Liability Regime for Biodiversity Damage: The Nagoya-Kuala Lumpur Supplementary Protocol, edited by Akiho Shibata. London: Routledge. http://www2.kobe-u.ac.jp/~akihos/en/grenoble_docs/30Shibata_intro2013.pdf
Smith, Frances B. 2000. The Biosafety Protocol: The Real Losers Are the Developing Countries, Washington, DC: National Legal Center for the Public Interest. https://www.heartland.org/publications-resources/publications/the-biosafety-protocol-the-real-losers-are-developing-countries
Tagliabue, Giovanni. 2016a. The Precautionary principle: Its misunderstandings and misuses in relation to “GMOs”. New Biotechnology, 33:437-439.
Tagliabue, Giovanni. 2016b. The necessary “GMO” denialism and scientific consensus, Journal of Science Communication, 15:1-11. http://jcom.sissa.it/archive/15/04/JCOM_1504_2016_Y01
Tagliabue, Giovanni. 2017. Product, not process! Explaining a basic concept in agricultural biotechnologies and food safety. Life Sciences, Society and Policy, 2017, 13:3:1-9. http://rdcu.be/pKBe
United Nations. 1992. Report of the United Nations Conference on Environment and Development, Annex I: Rio Declaration on Environment and Development. www.un.org/documents/ga/conf151/aconf15126-1annex1.htm
Ventura, Arnoldo. 2006. Do we still need the Cartagena Protocol? SciDevNet (website), 12 April. www.scidev.net/global/biotechnology/opinion/do-we-still-need-the-cartagena-protocol.html
WHO -World Health Organization. 2005. Modern food biotechnology, human health and development: An evidence-based study, Geneva: WHO. www.who.int/foodsafety/publications/biotech/biotech_en.pdf
WTO – World Trade Organization. 2006. Reports out on biotech disputes. www.wto.org/english/news_e/news06_e/291r_e.htm
WTO – World Trade Organization. 2010. Dispute DS291 European Communities – Measures Affecting the Approval and Marketing of Biotech Products, up-to-date at 24 February 2010. www.wto.org/english/tratop_e/dispu_e/cases_e/ds291_e.htm
Dr. Giovanni Molteni Tagliabue is an independent researcher based in Italy who studies and reports on the philosophy of life sciences and political science. He specializes in epistemological, socio-political and legislative aspects of agricultural biotechnologies and is the recipient of the 2017 Innoplanta Science Prize.
A version of this article was originally published at European Scientist and is republished here with permission. European Scientist can be found on Twitter @EuropeScientist































