“I was born with the murder gene.” That’s the chilling statement to the police by the murder-suspect in a true-life story featured in Esquire years ago about three generations of men: a convicted grandfather, a cop father, and a son accused of killing a friend.
The case inspired a book 2012 by William Landay, Defending Jacob, that became the model for Apple’s riveting series of the same name.
“As a writer, the idea that genetic destiny can override free will — that somebody might think,” said Landay in an interview. ‘Well, I can’t be blamed for my actions, because it’s in my genes’ — is haunting.”
 Haunting, yes, but is it grounded in science? Defense lawyers in an increasing number of cases are offering that as an ‘explanation’ in the wake of a starting Italian court case in 2010. The judge overseeing a murder case allowed the defense to conduct a ‘genetic susceptibility test’. It showed that the convict carried certain variants for the MAOA, CMT, SCL64A and DRD4 genes that have been associated with aggression. The judge decided to reduce the sentence.
Haunting, yes, but is it grounded in science? Defense lawyers in an increasing number of cases are offering that as an ‘explanation’ in the wake of a starting Italian court case in 2010. The judge overseeing a murder case allowed the defense to conduct a ‘genetic susceptibility test’. It showed that the convict carried certain variants for the MAOA, CMT, SCL64A and DRD4 genes that have been associated with aggression. The judge decided to reduce the sentence.
There are numerous compelling cases in the US justice system in which brothers, cousins or descendants from one family line have committed similar and very serious crimes. Identical twins Stephen and Robert Spahalski share not only their genetic makeup but also a chilling propensity for murder. Rather than collaborating in a crime, they embarked on separate murder sprees at different junctures in their lives. The murder orchestrated by Stephen at the age of 16 strikingly mirrors the one Robert committed three decades later.
How can we weigh the relative importance of cultural and environmental factors like socio-economic status or upbring versus the immeasurable role of genes?
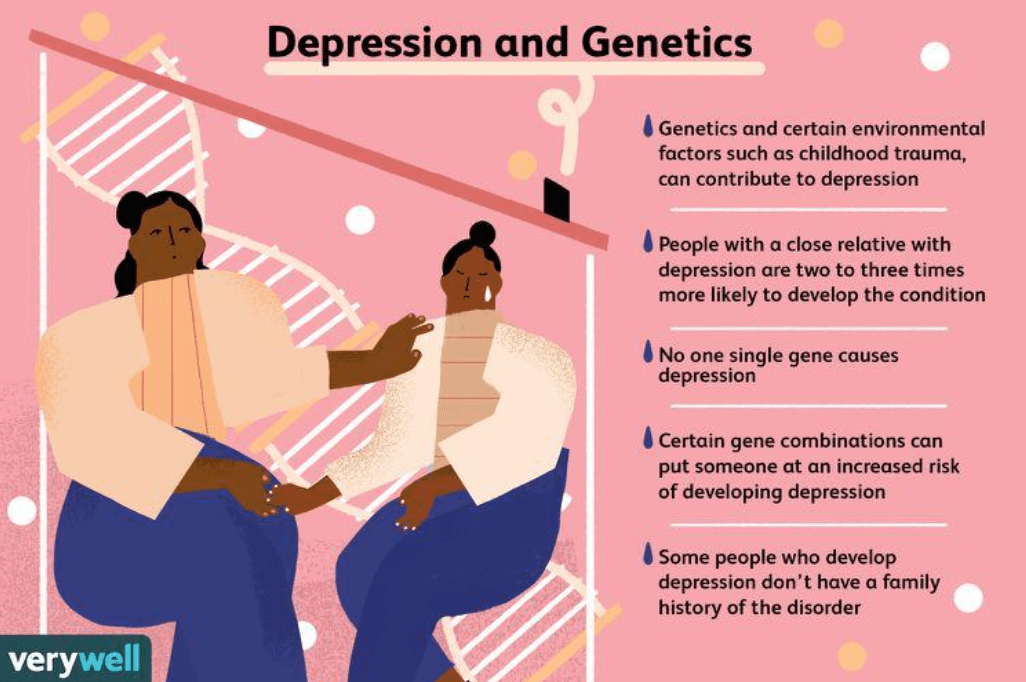
Mental health and inheritance
We know the influence of genes on extreme behavior is critical. A meta-analysis of 24 studies suggests as much as 50% of the total variance in aggressive behavior has genetic roots.
It’s consensus, based on significant measure from decades of study from the decades-long Minnesota Twins Reared Apart Study that identical twins raised separately often exhibit very similar behaviors across a wide spectrum, such as a propensity to smoke, nervous traits, religiosity, intelligence, and even the choice of car we drive or the hair color of the person we couple with.

Knowing that genes play such a critical role, it’s intriguing to wonder whether gene editing holds a key to addressing mental health struggles? It’s an enticing idea. Even setting aside enormous ethical concerns, scientists would have to navigate the enormous complexity of the brain. Studies on rats found that editing receptor genes linked to aggressive behavior yielded, in some cases, the exact opposite of what they expected to happen.
“This suggests a startling conclusion,” said Georgia State University Regents’ Professor of Neuroscience H. Elliott Albers co-led the research team. “We don’t understand this system as well as we thought we did. The counterintuitive findings tell us we need to start thinking about the actions of these receptors across entire circuits of the brain and not just in specific brain regions.”
And few of the tests on rodents and other animals are likely to yield actionable information about humans. The human cerebral cortex houses 1,000 times more neurons than that of a mouse. And the differences in the connectome between these species are still poorly understood.
Mental health challenges arise from a blend of genetics, environment, and experience, resisting simple solutions. The notion that gene editing could seamlessly rewrite the code of our mental well-being is enticing, but this promise isn’t within immediate reach. While gene editing is appealing as a solution to addressing mental issues, its realization will be a journey of careful steps.
What role can gene editing and biology-based solutions play in the near term?
Many conditions common illnesses carry a large genetic component. Bipolar disorder, for instance, is one of the most genetically influenced psychiatric conditions, with 70-90% of cases attributed to genetic factors. Polymorphisms in CACNA1C, ODZ4, TRANK1, GNG2, ANK3, and other genes have been linked to an increased risk of bipolar disorder.
Schizophrenia is an estimated 70-80% heritable, which is why it often runs in families. The risk is markedly higher for individuals with a parent, sibling or offspring first-degree relative who has it.
Autism spectrum disorder (ASD), a neurodevelopmental condition usually present from birth, has been linked to 180 ‘loss of function’ gene variants, including MECP2, and SHANK1-3. These and other pleiotropic genes disrupt synaptic and neural network development, and neural stem cell growth, illustrating the intricate web of genetics underpinning these conditions.
Genome-wide association studies (GWAS) have uncovered genetic distinctions in individuals reporting mental health disorders. A Yale University study of 1.2 million people with serious depression, using data from 23andMe and other data banks, uncovered a web of genetic variations absent in control groups.
The researchers found that, like many mental health disorders, there is a complex combination of many genetic variants. “That’s why we weren’t surprised by how many variants we found, and we don’t know how many more there are left to discover,” said Professor Joel Gelernter, senior author of the study. “And we don’t know how many more there are left to discover — hundreds? Maybe even thousands?”
Intriguingly, other research connects depression not only to genetics but to sex. Researchers at McGill University in Canada analyzed genetic data from almost 370,000 individuals, showed that major depression is linked to different genes in males and females. Dr. Patricia Pelufo Silveira, lead author of the study, emphasizes the significance of their findings:
“This is the first study to describe sex-specific genetic variants associated with depression, which is a very prevalent disease in both males and females. These findings are important to inform the development of specific therapies that will benefit both men and women while accounting for their differences.”
These insights indicate that if genetics contribute to conditions like depression, the specific mutations might differ by gender. Simply put, your gender may influence your risk of mental health disorders.
As we unravel these complexities, another question arises: What about treatment-resistant depression? Could that be linked to genes as well?
Some individuals don’t respond to conventional treatments. At times, they’ve opted for controversial options such as electroconvulsive therapy (ECT).
“ECT has been the gold standard for treating severe depression for over 80 years, “says Amit Anand, director of Psychiatry Translational Clinical Trials at Massachusetts General-Brigham, “but it is also a controversial treatment because it can cause memory loss, requires anaesthesia, and is associated with social stigma.”
Maybe there could be a genetic-based treatment option? A National Institute of Mental Health study uncovered rare genetic variants linked to treatment-resistant depression in over 10,000 individuals.
Don’t exclude environmental and epigenetic factors
It’s crucial to note that DNA alone is not an absolute prerequisite for developing depression. Like most complex diseases, genes elevate the risk rather. Additionally, epigenetic changes, which alter gene expression without modifying the DNA sequence, can also play a pivotal role.
Epigenetics factors include the modification of DNA or associated proteins that influence gene expression without altering the inherited DNA sequence. DNA methylation can silence genes, while histone modifications can open or close access to DNA, impacting various biological processes across development and health. These changes are often linked to the onset of mental health disorders.
Multiple studies suggest that trauma’s effects can be inherited because of epigenetic changes. This inheritance doesn’t pertain to mental health disorders themselves but to events that trigger depression, anxiety, and post-traumatic stress disorder (PTSD).
A University of California study indicated that paternal trauma from US Civil War ex-POWs has spanned generations in some cases. An analogous scenario was observed in a University of Groningen study that examined descendants of individuals who experienced the Dutch famine of 1944-1945. These descendants exhibited altered DNA methylation patterns, potentially elevating the risk of metabolic disorders. In essence, there may be situations where the life your ancestors lived and the hardships they faced could negatively impact health.

Not all scientists embrace the belief that epigenetics plays that consequential a role in the onset of depression, nor that trauma is inherited. Translating laboratory findings into clinical applications, they say, is complex. That necessitates further comprehensive research before we can more definitively conclude that epigenetic modifications can be linked to specific disorders.
Gene-based treatment options
As the quest to identify innovative solutions to mental health challenges intensifies, the focus on manipulating genes has become more prominent. But it’s crucial to distinguish among differing but related treatment options: gene therapy, gene editing and epigenetic therapy.
Gene Therapy involves introducing functional genes into a person’s cells to replace or supplement defective genes. This technique aims to rectify the genetic root causes of disorders. For instance, if a specific gene mutation is linked to a mental health disorder, doctors using gene therapy might seek to replace the faulty gene with a functional version.
Gene Editing entails precisely modifying an individual’s DNA to alter, remove or insert specific genetic sequences. A widely known technique, CRISPR-Cas9, acts like molecular scissors to cut DNA at precise locations. This can potentially enable researchers to correct or modify genes linked to mental health disorders.
Epigenetic Therapy involves a different approach. Instead of altering the DNA sequence itself, physicians can modify the epigenetic marks on DNA or associated proteins. These marks impact gene expression, influencing which genes are active or silent. Epigenetic therapy aims to reverse abnormal epigenetic patterns that might contribute to current or future mental health disorders, without directly modifying the genetic code.
Navigating the challenges
Translating all this information into a real world, therapeutic application is the challenge here. The prospects of these revolutionary treatments have led to torrent of media articles unmoored from a realistic timeline. “Does gene editing hold the key to improving mental health?”was the question posed in a recent article in The Guardian, “Gene editing could reverse anxiety and alcohol-use disorder”, speculated Big Think. However, the future of mental health-focused gene editing technology is more distant than many articles suggest. As is so often the case with breakthroughs, the scientific community is not in agreement as to whether these tools could and/or should be used.
While gene editing holds promise for addressing genetic factors in mental health disorders, there are concerns about the precision of techniques like CRISPR-Cas9 in treating complex conditions influenced by multiple genes. Ensuring that edited genes yield the desired effects, while avoiding unintended consequences, also remains a formidable challenge.
At this stage in the evolution of CRISPR, the outcomes aren’t always straightforward. Interactions within the intricate web of cellular processes can lead to unexpected results. Targeting a specific gene may trigger ripple effects across other genes and pathways, adding layers of complexity to an already intricate challenge. There is also debate about whether embryos are the most likely candidates for such interventions.
Everyone agrees that gene-editing to address mental health disorders, particularly the penchant for violence, remains ethically, socially, and technically contentious.
“What we have here, of course, is the incredibly slippery slope where we are no longer talking about people’s diseases, but about what makes them individuals, notes Dr. Robert Sapolsky of Stanford Medical School
While manipulating genes shows promise in targeting underlying genetic factors, concerns about consent, unintended consequences, and stigmatization are intensifying. The ethical terrain is complex. And there are questions about long-term effects, equitable access, and unforeseen consequences that could impact future generations. Striking a balance between potential benefits and ethical and social considerations is pivotal in navigating uncharted territory.
Embracing complexity for better mental health?
As a society, we often seek “silver bullets” to solve our problems. Look at the current hysteria of interest around ‘miracle’ weight loss drugs like Ozempic and Wegovy. Mental health disorders defy such simplistic solutions. They resist reduction to singular causes or cures. While advances in gene editing offer hope that we may more effectively address mental health challenges, they are unlikely ever to be a standalone solution. Their origins are a tapestry woven from environmental influences, life experiences, neurobiological factors and the mystery of our DNA.
Sam Moxon has a PhD in tissue engineering and is currently a research fellow in the field of regenerative medicine. He is a freelance writer with an interest in the development of new technologies to enhance medical therapies. Follow him on X @DrSamMoxon































