The creations of Jurassic World may be fiction, but the process most commonly known as de-extinction certainly is not. Species such as the woolly mammoth and the American passenger pigeon may soon be brought back to life.
De-extinction can be described as a process in which an organism that has been extinct has been reborn, or alternatively a species created that greatly resembles an extinct species is born. In the public eye, this idea of bringing extinct species back to life is becoming increasingly more prevalent, as high profile discoveries such as Yuka, the most well preserved mammoth ever found in glacial ice, is drawing the media’s interest, along with the idea of cloning the animal from its incredibly well preserved tissue.
Other types of animals that have high interest in being brought back include the moa, the Carolina parakeet and the American passenger pigeon, to name a few. Another example of its increasing popularity can be seen through popular mainstream literature such as the National Geographic devoting a cover story to this area of science, as well as establishments such as the Revive and Restore Network gaining support from both TED and the National Geographic Society holding TED conferences further explaining the science and possibilities behind it.
As of yet, there is little to no papers published upon de-extinction as this is still a very new science. However, the underlying experiments which is incorporated into the process of de-extinction is well documented. These include processes such as Interspecies germ cell transplantation, which involves using primordial germ cells (PGCs) from one species and genetically altering them to produce gametes that create another species and putting it back into the host. This causes the germline of these chimeric animals to change to another related species and allows them to give birth to species that lie out with their own.
Another option is to use new gene editing techniques such as the CRISPR-Cas9 system to specifically target genes of related species of extinct animals and manually alter genes to express phenotypes that the extinct species would have. Both of these techniques will be explored in greater detail later in this essay. Finally using classic cloning techniques using nuclear transfer of intact nuclei of cells into an enucleated oocyte could also be an option if intact nuclei with intact DNA were in existence for an extinct species.
Although one can naturally get excited about the prospect of bringing back animals that were once lost to the world, this brand new avenue of science contains a number of practical problems of using cloning techniques, as well as a variety of ethical issues such as ecological benefit, bio-objectification concerns and indeed whether it is right to bring back extinct species at all if they were unfit to survive? In this essay, a critical review shall be used on both the science and the variety of issues that could inhibit the development of this science into a true reality in the very near future.
Germline manipulation
Primordial germ cells (PGCs) can be described as the initial precursors to the production of sperm and eggs. In most animals, the migration of the germ line from their somatic origins is one of the earliest events that occur during development. The fact that PGCs tend to occur separately from their gonadal end goal for migration and specification, this allows the removal of these PGCs and allow the genetic editing of the original PGCs. Also, it allows the reintroduction of PGCs from another species, which would then cause chimeric animals that would produce genetically altered offspring or indeed, entirely new species through reproduction with another altered animal of the opposite sex.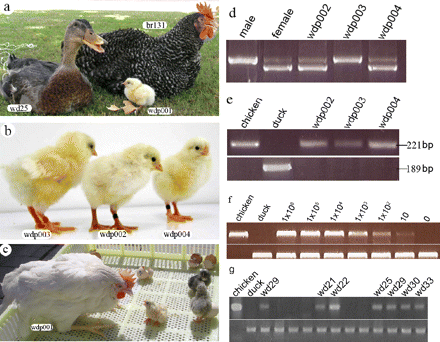
This process can be most easily carried out in avian embryos, as PGCs can be first identified in an extra-embryonic region known as the germinal crescent in the epiblast at around 18 hours of incubation. Then at around 50-55 hours of development, PGCs migrate and reach the developing gonad through the circulatory system, allowing the production of functioning sperm and oocytes (van de Lavoir, Diamond et al. 2006, Kuwana 1993). By removing the PGCs from one embryo and replacing them with PGCs from another, this would create a chimeric strain of that species with the ability to produce offspring from the second species.
An experiment conducted by Liu, Khazanehdari et al. aimed to create transgenic chimeric ducks (Anas domesticus) with chicken (Gallus gallus domesticus) PGCs that would go on to produce chicken offspring. There are several distinct phylogenic differences between the chicken and the duck. Ducks as waterfowl, belong to the order of Anseriformes, whilst chickens belong to the order of Gallinformes. In order for these chimeric ducks to produce chick offspring, the injected PGCs would be required to migrate to the gonads of the duck embryo, proliferate and then successfully differentiate into functional gametes in the gonads of the duck.
It was shown that chicken DNA had reached the gonad of the developing duck embryo a week before hatching by using specific chicken and WT probes via in-situ hybridisation. However, upon assessing the duck semen, it was seen that only 20.2 percent of total sperm produced by the ducks were of chicken origin, which naturally inhibits the fertility of the chimeric duck. Female chickens were then artificially inseminated with the chimeric duck semen, which produced two pure chicken embryos out of 367 eggs, giving a fertility rate of 0.8 percent and reached full maturity after four months (see Figure 1).
Albeit the experiment was a success in terms of producing an interspecies offspring, the frequency at which viable embryos were born was incredibly low. This could be due to the distance in evolution between the duck and the chicken, being from entirely different orders in the evolutionary timeline. As a result, exogenous germ cells can prove incompatible with Sertoli cells due to the differences in microenvironmental interactions between the duck and the chicken in this case, severely inhibiting the frequency of successful fertilisation. In a high output animal such as avian birds for eggs, a positive outcome of this type of experiment clearly occurs fairly swiftly. However if the same methods were used in animals that produce less offspring such as large mammals, it could prove far more difficult at such a low fertility rate as shown here. Naturally, the closer related a species is to the other inevitably improves its chances, but there is still the problem of the incompatibility between any two species when you integrate different cell lines of any sort.
Another problem with using non avian PGCs is the ease of the extraction of PGCs. For example, mouse PGCs can be found at the posterior end of the primitive streak and in the allantois, a far more difficult area for PGC extraction compared to the epiblast, as well as the obvious problem of then putting the chimeric embryo back into the mother’s uterus for in utero development to occur. However, when successful, this method of germline manipulation does have the immediate advantage of propagating endangered species. For example, the endangered Houbara Bustard was successfully produced in chimeric chicken producing bustard sperm.
Using similar analysis techniques, it was shown that out of 45 Houbara eggs, one surviving male live born Houbara bustard, shown to be of pure line. Although it encountered similar difficulties as the chicken-duck experiment in terms of fertility, the fact that an endangered species of bird was being produced in chickens – an animal that can produce fertile eggs non-seasonally throughout the entire year is of considerable advantage to the preservation of the Houbara buzzard species.
In terms of its effectiveness in its use to bring extinct species back from the dead, this method would prove useful in bringing back avian species such as the American passenger pigeon. By removing the PGCs from the most related species of pigeon to the passenger pigeon, and then removing the DNA or nucleus from the PGC and replacing it with intact DNA or nuclei of the passenger and reintroducing it to the host animal could in theory produce a chimera capable of creating pure line passenger pigeons. However the survivability of the altered PGCs could also be affected by the introduction of exogenous DNA into the cells, which could cause further migratory and proliferative problems on top of the initial downfalls of this type of experiment that has been covered previously. This type of alteration might be better suited to genetically editing the PGCs to maintain their initially endogenous status, presuming that the alterations made to the PGCs to turn them into something similar to the species in question that was to be resurrected doesn’t affect its ability to function as a PGC.
CRISPR/Cas-9 genome editing
Instead of using a de-extinction method that requires the insertion of an existing genome, it could be possible to alter the DNA of highly related surviving species and editing it to create an animal that is genetically identical to an extinct species. Now with the introduction of the CRISPR-Cas-9 (clustered regularly interspaced short palindromic repeats) genomic editing technology, we can accurately delete, insert and manipulate genes endogenously in any organism one chooses. Using this targeted genome editing technique, we can use customisable Cas9 nucleases allowing for the targeted deletions, insertions and sequence changes in nearly all organisms and cell types.
For any form of targeted genome editing, the creation of a double stranded DNA break (DSB) is vital for the genomic locus to be modified. When this occurs using techniques such as CRISPR, the break can be repaired via two methods: non-homologous end joining (NHEJ) and homology directed repair (HDR). Using NHEJ, whereby the break in the DNA leaves exposed ends of DNA on the broken strands can be used to add insertional/deletion mutations of varying lengths, thus disrupting the reading frame for a coding sequence or binding sites for trans-acting factors in genetic sequences such a promoters and enhancers. HDR repair uses specific point mutations in desired sequences through the recombination of target sites using exogenous DNA templates. Before CRISPR became the mainstay in genomic editing in 2013, zinc fingers (ZFN) and transcription activator like effector nucleases (TALENs) but each had a variety of advantages and disadvantages.
CRISPR’s natural role in bacteria and archaea was as an adaptive immune system to defend itself from foreign nucleic acid such as viruses. Type II CRISPR systems use sequences from invading DNA between CRISPR repeat sequences which are encoded as arrays within bacteria (Fig. 2a). These CRISPR repeat arrays are then broken down further into smaller CRISPR RNA (crRNA). These small segments of RNA act as a variable sequence which is transcribed from invading DNA called a protospacer sequence as well as the rest of the CRISPR repeat. Each crRNA hybridises with a second section of RNA, known as the transactivating CRISPR RNA (tracrRNA). These two sections of RNA complex with Cas9 to then cleave target DNA sequences from 3 base pairs from short non coding sequence known as protospacer adjacent motifs (PAM).
An example of a genetic engineered model of this system incorporates the type II CRISPR system from S. pyrogenes for creating DB and genome editing. In the simplest form of the system, the Cas 9 nuclease and a guide RNA (gRNA) – a fusion of crRNA and tracrRNA – is incorporated into cells of a target organism such as an ECS for manipulation purposes (Fig 2b). Twenty nucleotide at the 5’ end of the gRNA act as the protospacer to direct Ca9 to specific target DNA site using classic base pairing rules. These sequences lie immediately 5’ of the PAM sequence that matches the form 5’-NGG. Therefore Cas9 will be directed to all areas of DNA of the form N20-NGG by altering the first 20nt of the gRNA to align with specific target sequences.
The result of creating DBSs is that in the repair of these breaks causes high fidelity in the original structure, causing random and generally undesirable mutations, lest the technique is being used for specific gene knockout. However, using Homology Directed Repair (HDR) techniques allows specific genetic mutations of choice. This process involves electroporating or injecting cells with Cas9, sub genomic RNA (sgRNA) and a single stranded DNA oligonucleotide which is homologous to the breakpoint site. Therefore, using a combination of both of these techniques, one can create any mutation or genetic change at any part of any DNA.
These very recent discoveries has opened up the possibility for a more indirect route in bringing back extinct species of animal. By comparing the genetic structure of a species that is extinct to its most closely related relative, we can see often the very small percentage of genes that have been altered over evolutionary time. By using CRISPR techniques alongside HDR, we can isolate the differing genes in question of living species in the zygote of any animal (e.g. an Asian elephant) and simultaneously reprogram the genome to take on the phenotype of an extinct animal (e.g. a wooly mammoth). Allow gestation to take place and upon birth, we have a genetically engineered extinct animal.
This brand new technique that is barely a year old has revolutionized genetic engineering dramatically, and the possibilities beyond just this topic are incredibly far reaching; from eradicating diseases such a malaria to breaking down antibiotic resistances which is becoming an ever increasing problem. This technique is particularly useful if the DNA sample from the extinct species has become fragmented over time and would not be suitable for direct transfer through a cloning process or a PGC manipulation. However, the question remains should a genetically engineered mammoth really be considered a mammoth, even if the majority of genetic differences between it and the Asian elephant were altered? Indeed artificially creating new life based of a deceased model could really be referred to as true de-extinction is a matter of debate in many circles.
Cloning techniques
From practically brand new technologies in CRISPR to a technique that has remained relatively unchanged since its invention, the practice of cloning is still very much a developing science even now. From the first uncontested mammalian clones of lambs conducted by Willadsen in 1986 by the process of nuclear transfer, this relatively simple concept has remained more or less the same when used in the present day.
The most famous case of successful cloning came in the form of Dolly the sheep, produced by Wilmut in 1998 for the Royal Institute in Edinburgh, Scotland. In this classic experiment of the form, the donor culture of cells was derived from mammary tissue, and was initially deprived on nutrients to slow down cellular processes. Then a Scottish Blackface ewe provided an oocyte that was removed during metaphase II. The spindle fibres and chromosomes were removed using a glass micropipette and the donor cell was placed adjacent to the now enucleated oocyte. An electrical stimulus was then presented to the two cells, which initialised the fusion of the oocyte and the donor cell. From there, the oocyte was fertilised, placed back in the mother’s uterus and gestation was allowed to occur. From there, Dolly was born, to which allelic DNA microsatellite analysis confirmed that Dolly belonged to the same Finn Dorset breed of sheep from which the donor cell derived from. Dolly was a reproductively competent clone who went on to produce 3 offspring.
From the initial beginnings of Dolly also gave birth to the idea of transgenic cloning, which was successfully achieved by Schnieke, Kind et al. in 1997, who successfully trans infected foetal sheep fibroblasts in culture with the human gene for clotting factor IX, alongside a regulatory gene to restrict its expression to the mammary gland, alongside a gene for neomycin resistance to concentrate the culture of genetically modified fibroblast tissue. These fibroblast tissues were then fused with an oocyte which gave birth to Polly the sheep, who successfully produced human clotting factor in her milk, giving way to new advances in increased drugs and other biological products in transgenic mammals over those initially produced in bacteria.
As it has been proved several times after these initial experiments since then, it is become an undeniable fact that transgenic mammals, and thusly all animals, can be successfully engineered. On this account, should a cell from an extinct species be preserved to allow the cellular fusion of itself with an enucleated oocyte of its closest common ancestor, then in theory, it could give birth back to the species.
Following on from this idea, a variety of experiment have been conducted in the pursuit of whether it would be possible to use somatic cell nuclear transfer (SCNT) and genomics to bring back extinct species. These experiments include the successful birth of offspring using SCNT using frozen mouse cells without a cryoprotectant, which then caused follow up experiment where cloned offspring were produced from ESCs from frozen mice bodies. The pinnacle of this initial investigation over whether cloning was viable came from the successful transgenic mouse created which contained Col2A1 enhancer gene from the already extinct Tasmanian tiger Thylacinus cynocephalus) whose DNA has been preserved in ethanol for over 100 years. All these experiments give excellent evidence that as long as DNA has been preserved in some form (in these cases, in cryostasis or in alcohol) then the DNA at least can be resurrected in other animals.
After so many experiments in this field, the first successful cloning of an extinct animal was achieved in the form of a Pyreanean ibex (Capra pyreaica pyrenaica) through SCNT techniques from cells of the last living Ibex (Folch, Cocero et al. 2009). Skin biopsies of the ibex were taken, expanded in vitro and preserved in liquid nitrogen. Using SCNT methods outlined above, a female ibex was born from a domestic goat, and however it died within minutes of being born due to severe respiratory distress. The post-mortem report showed that the new-born had an atelectasis, as well as a lump on the left long, showing the cause of death. However, nuclear DNA microsatellite analysis as well as mitochondrial DNA analysis confirmed that the new-born did spawn from the last Pyrenean ibex.
The occurrence of severe developmental abnormalities in cloned animals is an unavoidable observation when assessing the technique’s effectiveness. For a start, SCNT effectiveness was assessed to be 4 percent, being the number of newborn animals in good health for every reconstructed embryo, regardless of the species of origin. In Brisville’s study on respiratory illness in cloned cattle, nine out of 18 calves developed respiratory diseases within 12 hours of birth, whilst six developed respiratory illnesses 24 hours after birth with only three appearing healthy.
On top of this, due to the nature of using adult genetic information in the creation of new life, the length of telomeres on the chromosomes are significantly shorter compared to a natural birth, irrevocably reducing the lifespan of the cloned organism. Also, large offspring syndrome (LOS has also been observed in cloned cattle and sheep as well as a variety of other defects across the board in regards to cloning. It is clear that in regards to regularly successful cloning we are still very much far away from a solution that can avoid the myriad of problems of using SCNT techniques in conservation and the resurrection of extinct species.
What does future hold?
It is apparent that huge advances in de-extinction have occurred over recent years, moving from science fiction to a very apparent reality, with attempts already being made to bring back animals such as the ibex. However there is clearly still a significant amount of fine tuning required to improve the possibilities of having successful resurrections. In the case of PGCs, this is proving to be potentially the most reliable source of conservation technique out of the three that this article has covered, but has only really been seen working with genetic material taken from living organisms, as well as the fact the success rate in which successful fertilisation can occur is really very small to be used in organisms that naturally produce less offspring; not to mention the complications of using this type of technique on animals who require this process to occur in utero.
CRISPR/Cas9 technologies appear to be a highly promising new technique in multiple areas of medicine, and definitely has the ability to artificially create life based on extinct species. However the technique is still in its infancy as well as the fact that should extinct species be created via this method, it could be argued that the end product of the experiment could only be considered a genetically engineered version of its closest living relative and could not give a true understanding of how the extinct species lived when it were alive. In terms of using SCNT techniques, its success rates are also very low, with added issues in regards to the high probability of developmental defects as well as their shortened lifespan due to shortened telomeres. Is it right to bring back an animal (in which most cases, were killed due to human involvement) to only expose it to a stunted life?
The generalised ethical issues regarding de-extinction are also vast. It is natural to feel responsible for animals such as the American passenger pigeon, one of the most populous birds in the world, were drove from the world by human interference, but naturally we will then have to make sure we do not become blasé in regards to the conservation of species. For those animals that died out simply due to natural selection, such as the mammoth, is it really right that we bring back an animal in which its chances of survival were minimal even before humans began to dominate the Earth and inevitably change the environment around us to become possibly less inhabitable? Is there an ecological benefit for bringing back an animal that could help better the planet? Or is it a simple matter of bio-objectification and human curiosity that is driving us to defy nature at its most basic level.
All these questions need to be considered on top of improving the techniques in place currently before we can really consider bringing back animals that have become extinct, however the fact that an animal as legendary as the woolly mammoth possibly being alive and well in this lifetime is something that is undeniably exciting and very, very possible.
Acknowledgements: I would like to thank Ben Novak and his team at Revive and Restore for sending me several articles on CRISPR techniques as well as the use of PGCs, which is the basis of their continuing work to bring back the American Passenger Pigeon to the skies.
Jamie Boyle, bachelor of science in Human Embryology and Developmental Biology from the University of Aberdeen. Current Masters in Research student in Intergrative Mammalian Biology at the University of Glasgow. Follow Jamie Boyle on Twitter: @jab93































