Following the submission of [an] application …. from Monsanto Company (referred to hereafter as the applicant), the Panel on Genetically Modified Organisms of the European Food Safety Authority (referred to hereafter as GMO Panel) was asked to deliver a Scientific Opinion on the safety of genetically modified drought‐ and glyphosate‐tolerant and insect resistant maize …. referred to hereafter as ‘five‐event stack maize’) and its subcombinations
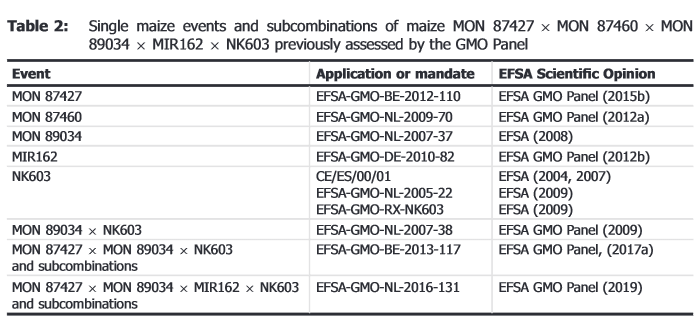
The GMO Panel previously assessed the five single maize events and eleven of the subcombinations and did not identify safety concerns. No new data on the single maize events or the 11 subcombinations that could lead to modification of the original conclusions on their safety were identified.
The molecular characterisation, comparative analysis …. and the outcome of the toxicological, allergenicity and nutritional assessment indicate that the combination of the single maize events and of the newly expressed proteins in the five‐event stack maize does not give rise to food and feed safety and nutritional concerns.
The GMO Panel concludes that the five‐event stack maize, as described in this application, is as safe as and nutritionally equivalent to its non‐GM comparator and the non‐GM reference varieties tested. In the case of accidental release of viable grains of the five‐event stack maize into the environment, this would not raise environmental safety concerns.
…
The GMO Panel concludes that there is a very low likelihood of environmental effects resulting from the accidental release of viable grains from the five‐event stack maize into the environment.
Since no new data on the eleven subcombinations previously assessed that would lead to a modification of the original conclusions on their safety were identified, the GMO Panel considers that its previous conclusions on these maize stacks remain valid.
Read full, original article: Assessment of genetically modified maize MON 87427 × MON 87460 × MON 89034 × MIR162 × NK603 and subcombinations, for food and feed uses, under Regulation (EC) No 1829/2003 (application EFSA‐GMO‐NL‐2016‐134)































