The story of CRISPR as a gene editing tool starts in 2012. In a pioneering work published in Science, a research team lead by Emmanuelle Charpentier and Jennifer Doudna showed that CRISPR/Cas9, a protein involved in bacterial immunity against viruses, can become a programmable DNA-cutting tool. Even though it was not the first nuclease that recognizes specific sequences, it was the first that used DNA-RNA complementation. And its simplicity made CRISPR a favored tool for biologists across disciplines within the next few years.
Since the first discovery, CRISPR has evolved from a gene-editing tool into a tool-delivering platform. CRISPR-based tools include a version where the nuclease doesn’t cut the DNA (blocking the expression of the bound gene), a base-editor that doesn’t break the DNA but mutates a single DNA base into another, and a recent protein complex that inserts transposons into the genome in a programmable manner.
CRISPR applications extend to various fields and industries, but probably what gains most attention are the ones in medicine. The precision of the DNA recognition makes CRISPR a powerful diagnostics tool, particularly suited for quick detection in remote areas. But the real game changer is the potential to edit human cells and treat genetic disease. Given that more than 70,000 mutations are involved in disease or have health implications, the use of a powerful gene editor can improve the healthcare of most of the population. CRISPR is currently used in several clinical trials that target various different health conditions.
Despite its potential, CRISPR-based therapeutics is not ready for generalized used in clinics. There are two major problems that scientists need to address. The first one is the specificity. The nuclease recognizes a particular sequence with very high efficiency. But it may also recognize a similar DNA sequence and edit off-target, albeit at a much lower rate. Nevertheless, the clinical specialists need to cleverly design the recognition sequence and the time the nuclease is active to minimize the risk of off-target mutations. The second challenge comes from the way the CRISPR nuclease acts on the DNA. Cas9 introduces a break in both DNA strands. The cell’s repair mechanisms sense the structural damage and try to repair it. This repair is not error-free, and has again the risk of introducing mutations.
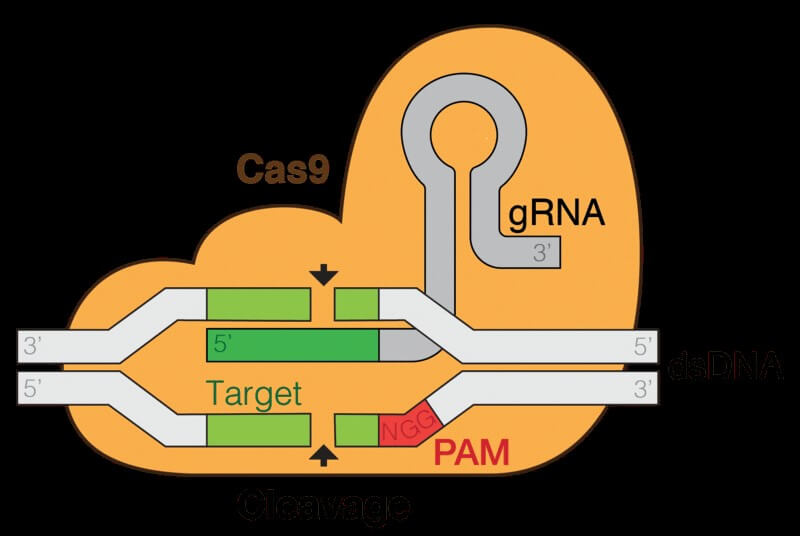
Prime editing is a CRISPR technique that aims to address both of the problems mentioned above. The Harvard researchers used a nuclease that cuts only one of the two DNA strand to minimize the structural damage done to DNA. The next step of the editing is where the real innovation takes place. The prime editing nuclease comes attached to a reverse transcriptase, an enzyme that synthesizes DNA using an RNA template. And as CRISPR uses RNA to guide itself to a sequence, it is not challenging to add the “repair” template to the same molecule. The result is a multifunctional complex that recognizes a sequence, attaches on it, and corrects it according to the template it contains within. For more details on the mechanism of action see the infographic released by the BROAD institute here.
The new editing technique has the capability to introduce specified changes to the DNA: mutations, deletions, and insertions of new base pairs. Liu’s team showed that prime editing works in vitro, in yeast, and in human cells. The unique mode of action minimizes mistakes, and the off-target mutations are significantly reduced compared to the “traditional” CRISPR/Cas9 methodologies. The flexibility of prime editing increases the scope of genetic disease that can be treated via CRISPR – up to 89% of the known mutations of clinical significance are a valid target according to the authors’ calculations.
These first results are very encouraging and the new technique will develop further. The next aims include the improvement of prime editing’s specificity and efficiency. The technique should be also tested in the hands of other research groups to ensure the reproducibility and robustness of the technique. Meanwhile, the companies Beam Therapeutics and Prime Medicine (both cofounded by Liu) join forces to explore the new technique and bring it to the market.
The advances in genome editing bring the promise of better genetic treatments available in the next few years. Many challenges still remain, especially when it comes to the technique’s safety, but now researchers and clinicians have tools unimaginable even five years ago. Of course, genome editing comes with several ethical implications. Is it OK to edit germline cells that will affect not only the patient but also their offspring? When do the potential risks of an intervention outweigh the health benefits? Will these new technologies be available to all patients or only to the select few that can afford the bills?
CRISPR is revolutionizing genetic engineering and is a good example of how human creativity can take a bacterial tool that exists for several million years and used it to revolutionize biological research. The upcoming years will show if the promise of precise gene editing holds true. If so, healthcare will enter a new era.
Kostas Vavitsas, PhD, is a Senior Research Associate at the University of Athens, Greece. He is also community editor for PLOS Synbio and steering committee member of EUSynBioS. Follow him on Twitter @konvavitsas































