The SARS-CoV-2 virus is threatening to surge again. In the past few weeks alone, a recent version, XBB.1.5, has quickly spread in the United States. As of the most recent data from the Centers for Disease Control, this variant makes up about three-quarters of all cases in the Northeast and 27.6% nationwide.
“It is the most transmissible variant that has been detected yet,” said Maria Van Kerkhove, the Covid-19 technical lead at the World Health Organization. But transmissibility, or infectiousness, is only part of the story.
The progression of a pandemic depends on several factors, including: (1) the characteristics of the pathogenic organism, primarily its infectiousness and virulence; (2) the state of immunity in the population from either vaccination or previous infection; (3) our ability to treat the infection; and (4) the willingness of the population to behave in ways that prevent infection.
The current state of all these variables is worrisome. But I’m getting ahead of myself.
Are US COVID control policies deficient?
The patchwork of policies and what some have derided as ‘moving the goalposts’ to respond to new developments is not surprising nor an indication of a public health failure. New data constantly become available, and circumstances change, and therefor so must our responses. The situation is somewhat like the way that our automobile navigation systems work. When you start the journey, the device provides an estimated time of arrival and plots the route, but that can change according to various unplanned occurrences — if you stop for gas or encounter an open drawbridge, for example.
The operative concept for managing the pandemic, or any medical problem, for that matter, can be summarized in a single phrase: minimizing the probability of adverse outcomes. But we, as individuals, public health officials and political leaders, have widely differing tolerance for risk and inconvenience, and the risk-related circumstances vary widely in different states and localities. One size doesn’t fit all.
Managing probability means reducing the chances of being exposed to an amount of the SARS-CoV-2 virus sufficient to penetrate the body’s natural and vaccine-induced defenses and cause an infection. At the same time, we must not impose measures that cause diminishing returns. The cure should not be worse than the disease. It can be a difficult balancing act.
Barriers to infection
Some of the barriers to infection are illustrated nicely in this figure below, the original version of which was created by Australian virologist Ian Mackay:
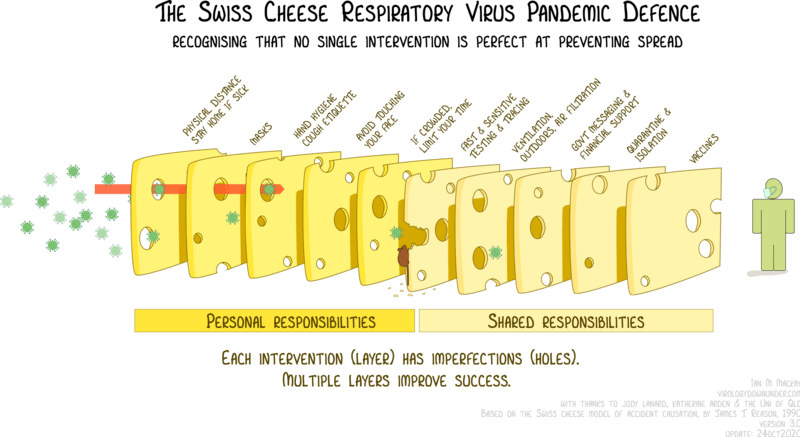
Each of these measures lowers the probability that an infectious dose of virus will find its way to your respiratory tract, and if it does, that it will cause an infection. Also, depending on the extent and effectiveness of vaccination, some of the other interventions could become superfluous, or at least less important. However, the protection afforded by vaccination wanes with time, especially as new SARS-CoV-2 variants arise that boast a higher degree of immune-escape.
Such strategies are the essence of preventive medicine. For example, many of us take drugs to lower our cholesterol levels or blood pressure to reduce the probability of cardiovascular disease, and get flu vaccine to prevent influenza, and workers with fiberglass wear masks to prevent inhalation of harmful small particles.
Survival of the fittest pathogen
Erecting barriers — biological, physical, and behavioral — to prevent infection is well understood. What is harder is predicting how the microorganism that causes the infection will evolve, and what responses will be required. There is a wide spectrum of possibilities, which are dependent upon … probabilities.
The SARS-CoV-2 virus has evolved constantly, and sometimes drastically, since its discovery. Every viral replication in every infection – of which there have now been billions – creates new mutants and new candidates for Darwinian evolution to evaluate them for “fitness.” The fittest survive and spread.
Understanding XBB.1.5
Just one month ago, the Omicron XBB.1.5, accounted for 1% of all US infections, meaning its penetration has increased some 27-fold. The trait that makes XBB.1.5 more infectious than its predecessors is that it is more “sticky” than its predecessors – that is, it binds more tightly to the site on host cells where it attaches and enters. It does not, however, appear to be more immune-evasive, which is good news.
There has been a sharp uptick of COVID hospitalizations in the U.S. since the beginning of December, offering a preview of what might be in store nationwide as XBB.1.5 continues to spread. Even before the dominance of XBB.1.5, COVID hospitalizations had begun to trend upward nationwide.
There’s an important lesson from the pedigree of XBB.1.5. It evolved from XBB.1, which had evolved from XBB, an Omicron subvariant that emerged in India last August and quickly became predominant there and then elsewhere in Asia. Here is a map of the SARS-CoV-2 variants and subvariants prior to the appearance of XBB.1.5:
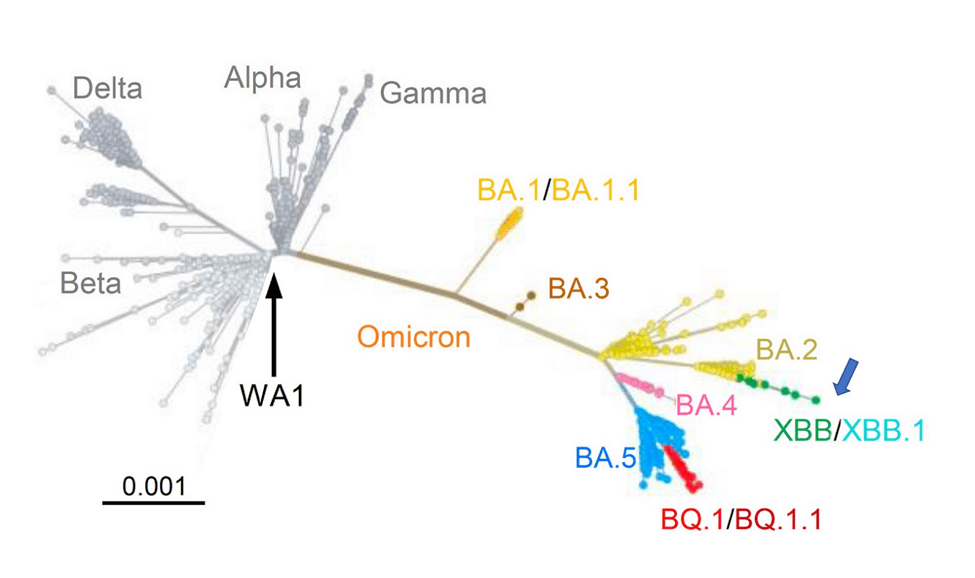
But the most striking thing is that XBB didn’t simply arise as the result of point mutations in the RNA; rather it is the result of a recombination of two descendants of the BA.2 variant. Swiss virologist Dr. Emma Hodcroft has made a useful diagram that illustrates what that means:
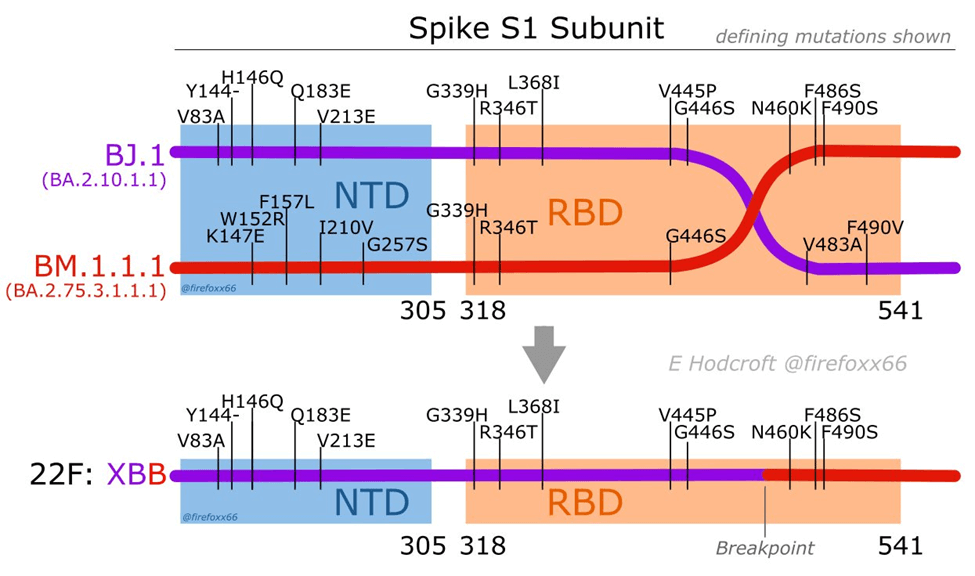
It might look complicated, but in the upper part of the figure we need only focus on the horizontal purple and red lines, which represent the RNAs that comprise the genome of two discrete subvariants of the BA.2 variant before recombination occurred. The bottom part of the figure depicts the genome of the new virus, XBB, that resulted from recombination (that is, breakage and reattachment, or what Dr. Hodcroft calls “template switching”). XBB is comprised of genetic material from both of the “parents,” which is shown by the line being both purple and red, joined at the “breakpoint.”
Dr. Hodcroft described the process this way:
When a virus copies itself, it can ‘template switch’ between different genomes floating around. If those are all the same, the end product isn’t different! But if distinct lineages are there at the same time, we can spot these ‘chimera’ children – recombinants!
This is somewhat similar to what happens when an animal is infected by two different flu viruses, and inside the animal they exchange RNA fragments to create a new, more dangerous virus which goes on to spread.
Lowering the probability
The important takeaway: For the recombination event to have occurred, the two parental viruses must both have infected a person and have been present in the same cell simultaneously for enzymes to have broken and reattached portions of the two genomes.
That scientific observation has important implications for public policy: To lower the likelihood of more dangerous subvariants arising in this way, we should strive to lower the probability of such co-infection events. We do that by decreasing the number of infections that occur, and we do that by applying the old 2020 rallying cry: “Flatten the curve.”
How? By judiciously applying the various “slices” of the Swiss Cheese Model above, of course — masks, vaccines, good indoor ventilation, avoiding high-risk situations, and so on — all of which are only being tepidly recommended by public health officials and have been largely abandoned in the U.S. There is hardly a mask to be seen on public transportation, in airports and airplanes, and in supermarkets and big-box stores. The bivalent booster vaccination rate for Americans five years of age and over is a dismal 15.4%.
As a result, hospitalizations, deaths, and long COVID are all trending upward. And the pandemic rages on…
Henry I. Miller, a physician and molecular biologist, was the founding director of the FDA’s Office of Biotechnology. He is the Glenn Swogger Distinguished Fellow at the American Council on Science and Health. Find Henry on Twitter @henryimiller































