Traditional Agrobacterium strains deliver transfer DNA, or T-DNA, into plants, including crops, and integrate it into the plants’ genome. This can create a plant that expresses traits, such as improved drought resistance or better nutritional content, that are valued by growers and can be useful to industry. However, T-DNA is permanently integrated into the plant genome, creating plants labeled “transgenic.” Transgenic plants can be either highly regulated or outlawed.
Purdue biologists have developed Agrobacterium strains that deliver T-DNA so plants can still be modified to express valued traits, but they are not transgenic. This means traditional methods to remove T-DNA aren’t needed. The strains were created by Stanton Gelvin, the Edwin Umbarger Distinguished Professor of Biology, and Lan-Ying Lee, research scientist, in the Department of Biological Sciences of Purdue University’s College of Science.
Gelvin said these VirD2 mutant Agrobacterium strains can carry T-DNA that delivers and expresses genome engineering reagents, such as CRISPR-Cas. Although the plant genome may be altered, no transgenic plant is created.
“T-DNA delivered by Purdue-created Agrobacterium strains disappears from the plant nucleus because it is eventually destroyed by nucleases – naturally existing enzymes that degrade DNA – or it is ‘diluted’ out of the plant nuclei as the cells divide,” Gelvin said.
The traditional method to remove integrated T-DNA from transgenic plants is to sexually cross a transgenic plant with a nontransgenic plant. Gelvin said this method has drawbacks.
“Crossing can be time-consuming and costly, generally requiring several generations of plants,” Gelvin said. “This isn’t feasible for plants with long generation times, such as many trees used for fruit or production of lumber, or crops that are normally vegetatively propagated, such as potatoes, sweet potatoes and bananas. These Purdue-created Agrobacterium strains avoid these drawbacks.”
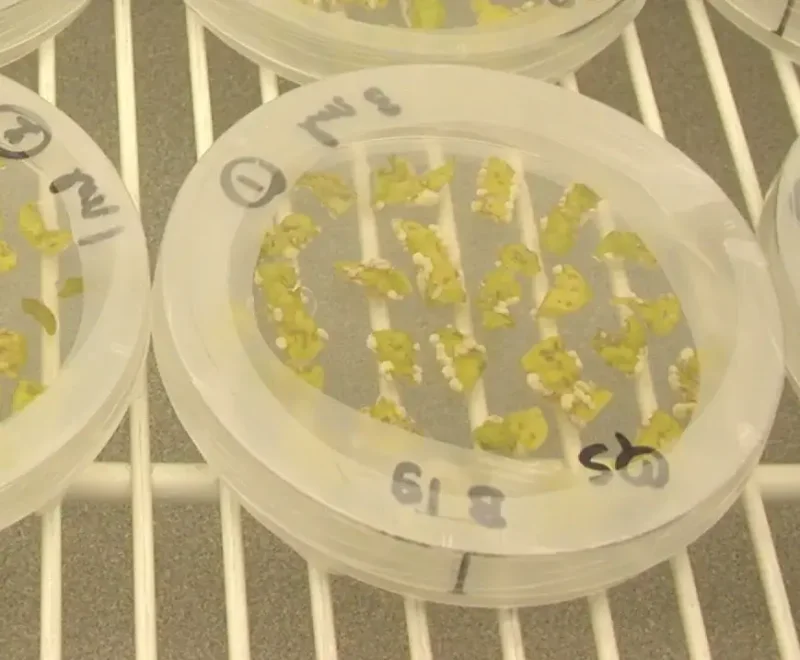
Gelvin and Lee have successfully used their strains on preliminary genome engineering of model plant species. Their altered Agrobacterium strains mutated a tobacco phytoene desaturase gene, encoding an enzyme involved in chlorophyll synthesis, at 50%-80% of levels mutated by normal, wild-type Agrobacterium strains, but without generating a transgenic plant.
“There are numerous traits that scientists and companies would like to introduce, but our strains are perhaps best used for genome engineering of any gene,” Gelvin said. “Lan-Ying and I continue to conduct additional experiments as we try to make these strains easier to use in academic laboratory and industrial settings.”