Does greater prior exposure to pathogens, including other recent coronaviruses, help explain why Africa is a COVID-19 cold spot, despite endemic poverty and a woeful health infrastructure? Conversely, in the more sanitized surroundings of the more developed world, people’s immune systems may be insufficiently ‘trained’ to cope with novel disorders, such as COVID-19 — what’s known as the ‘hygiene hypothesis’.
According to this thesis, the very conditions expected to cause the rapid spread of the virus may instead have primed the immune system of native Africans to better resist this latest disease, along with antibodies gained through exposure to numerous infectious ailments since childhood.
This is also how population-wide ‘herd immunity’ is naturally selected across evolutionary time, with those genetically more resistant to specific diseases surviving and reproducing at greater rates than the more vulnerable. It’s known that the Black Death epidemic didn’t just wipe out millions of Europeans (1/3 of the population) during the 14th century; it left a permanent, protective marker on the human genome, favoring those who carried immune system genes, who passed them on over subsequent centuries.

Certainly, with disease a long-recognized feature of the living world, it is no surprise it is a major factor in Charles Darwin’s theory of natural selection. Darwin himself had seen the devastating effects of disease on indigenous peoples during the voyage of the Beagle, and it featured in his later account of human evolution (and extinction), Descent of Man.
Disease is also central to the convincing ‘Guns, Germs & Steel’ thesis advanced by biogeographer Jared Diamond. According to Diamond, after the advent of agriculture in Eurasia (c. 10,000 years ago), the denser populations of the new farming societies were ideal breeding grounds for novel diseases (a process exacerbated by close contact with disease-bearing domesticated animals). Over millennia, newly-emerging diseases periodically swept along Eurasia’s trade routes, genetically-selecting for disease-resistance among the surviving populations.

Most relevant here is the grim historical consequence of this evolved genetic immunity among some populations but not others. In Diamond’s estimation, more than 90 percent of the indigenous peoples of the Americas — never before exposed to the deadly contagions of Eurasia—were wiped out within decades of the arrival of European colonists and invaders.
The New World disease apocalypse mirrors others that have plagued humankind for millennia. Recent genetic research suggests that Europe’s original Neolithic population may have been displaced by disease-resistant peoples from the east, 5000 to 6000 years ago. Once again, evolved population differences may have proved key, with newcomers’ longer exposure to the plague bacterium, Yersinia pestis, likely providing partial immunity not shared by the existing European populations. (The Black Death, the Spanish Flu and now COVID-19 are well-known historical examples of this process.)
There is also tantalizing, emerging evidence that ancient coronaviruses drove natural selection for disease resistance in East Asia between 25,000 and 5,000 years ago, with “[t]hese adaptive events … limited to ancestral East Asian populations, the geographical origin of several modern coronavirus epidemics” [emphasis added]. Indeed, our new-found abilities to probe the secrets of ancient DNA also allow us to search even further back into human prehistory — and into the divergent evolutionary pathways followed by a distant human cousin, the Neanderthals.
Do Neanderthal genes increase the risk of COVID-19?
The answer is ‘yes’. In fact, the presence of a Neanderthal gene is the single biggest genetic risk factor for the novel coronavirus, roughly doubling the likelihood, according to a June 2020 study by researchers in Germany and Japan, Hugo Zeberg and Svante Pääbo.
In follow up research published in February, they identified one haplotype that “is present at substantial frequencies in all regions of the world outside Africa. Given that Neanderthals were a branch of hominids seemingly confined to western Eurasia, sub-Saharan African populations never interbreed with this sister-species. This particular stretch of Neanderthal DNA is carried by around 50 percent of South Asians, 16 percent of those of European descent, but not in any native Africans.

Illustrating the complexity (and ironies) of evolution, the researchers suggest that these genes may have protected our distant cousins against pathogens found in their ancient environment, and were hence retained in some Eurasian populations subsequent to human-Neanderthal inter-breeding. Now, however, these same genes may have a detrimental effect with the different COVID-19 pathogen.
In the words of the researchers, the immune response for carriers of these Neanderthal sequences “might be overly aggressive,” leading to potentially fatal reactions in those who develop severe COVID-19 symptoms. This explanation is analogous to the genetics of HIV susceptibility, with ancient-acquired advantageous genes now proving disadvantageous. Importantly, this could also be true for any human population, African or non-African, that may show a different response to any disease.
Reviving an egregious, racist, un-scientific concept?
All populations, and individuals, are a mishmash of unique genes acquired from ancestral populations. Yet while the evidence of divergent evolution between distinct human groups continues to accumulate, such evidence also continues to cause disquiet.
Is it suspect — even racist—to explore the relationship of disease to population? Although the historically problematic term of ‘race’ is considered a controversial subject, geneticists have long recognized genetic differences among population in disease proclivities. Renowned population geneticist Neil Risch and colleagues addressed this issue years ago in a seminal paper, “Categorization of Humans in Bio-Medical Research: Genes, Race and Disease, writing:
… we believe that identifying genetic differences between races and ethnic groups, be they for random genetic markers, genes that lead to disease susceptibility or variation in drug response, is scientifically appropriate. … Every race and even ethnic group within the races has its own collection of clinical priorities based on differing prevalence of diseases.
That acknowledged, exploring this issue risks bringing opprobrium down on those who raise it. As Jared Diamond has written, “few scientists dare to study racial origins, lest they be branded racists just for being interested in the subject”. Given the racist history of biological beliefs about human differences (and, in this case, the xenophobic backdrop to the current COVID-19 pandemic), it is a topic that must be addressed with care, if directly and honestly.
As we’ve repeatedly make clear, apparent differences in disease risk among populations need not have direct biological causes. With COVID-19 it is already well-established that age, obesity and other pre-existing health problems bear on the severity of the disease, and these factors are unevenly distributed among different racial groups.
Some risk indicators are more important than others; in the US, for example, age does not appear the key determining factor in the impact of COVID-19 across sub-populations; rather it’s more about ‘race’ and economic class. The most common age for White Americans who have contracted the coronavirus is 58 years; for African Americans it is 27. Yet, despite being much younger on average, Black Americans are much more likely than White Americans to contract, be hospitalized by, or die from the novel coronavirus.

The same is true among indigenous American, Hispanic communities, and Pacific Islanders in Hawai’i. The major mortality predictor so far is social inequality — deprivation, marginalization, inadequate access to health care, and the like. Some of those factors have also contributed to higher rates of co-morbidities among COVID-19-vulnerable minority populations.
Genetic and social factors should be examined simultaneously
What does all this add up to? It’s complicated. There are appropriate concerns that incorporating population-based genetic explanations may trivialize the complexity of interaction between genes, environment and society. Such criticism is well taken, yet it cuts both ways: just as over-emphasis on genes may ignore crucial environmental factors, so too might a solitary focus on environment overlook relevant genetic influences. The historical misuse of facile ‘race’ concepts to support odious hierarchies does not mean we should ignore possible genetic influences on the spread of the virus.
Unfortunately, a common but mistaken belief among those suspicious of genetic research is that those who advocate that population genetics should play a central critical role in assessing disease susceptibility embrace the discredited belief that genes determine outcomes. By this facile argument, if genes are implicated in susceptibility/resistance to coronavirus, they must override everything else.
But no serious scientist studying this complex issue are determinists; that’s a notion decades out of date. Citing the tragically banal fact that that Americans of primarily African ancestry are more likely to die from COVID does not come close to ‘disproving’ the very likelihood that the genetics of those of African descent might have certain ‘protective’ genes.
A more nuanced and non-deterministic assessment points to the continuous interaction between genes and environment. If genetics play a role in the apparent partial-immunity of native Africans to the coronavirus, similar influences for those of mixed African ancestry in the US and elsewhere could still be swamped by an onerous social environment faced by Blacks and other minorities.
The subtle give-and-take of genetics and the environment may inform the experience of Africans of Bangladeshi descent. While UK-based Bangladeshis — a socially-disadvantaged minority group — are twice as likely to die from the coronavirus as the general British population, the COVID-related death totals in Bangladesh (8,000 deaths), is plummeting.
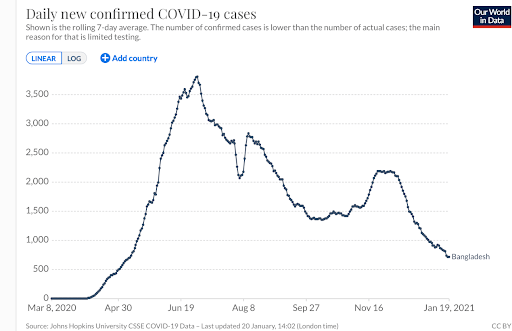
But the Bangladeshi example is also significant because it illustrates the complexity of the issue and conflicting narratives about genetics and the coronavirus. Bangladesh as a country is doing better than many countries which is both surprising and not so. The country ranks 57 out of 152 globally. The US ranks 143.

The average age (25.7) is comparatively young. Like Africans, Bangladeshi might have been expected to have received some protection based on the “hygiene hypothesis.” But clearly there are myriad other factors at play, including the intriguing fact that Bangladeshi carry the gene cluster implicated as likely increasing the risk from COVID-19 (more than half the population, 63 percent, carry at least one copy of the risk haplotype). The interaction among genes, environment and disease is very complex, eluding facile, definitive answers.
An ethical imperative
We already know environmental inequalities impact at-risk racial populations (such as those defined, in the US, as African Americans, Hispanics, Native Americans, and Pacific Islanders. This does not negate the likelihood that ancestry influences disease prevalence within these groups or in response to treatments. But this is an empirical question that can be answered only through studying group differences, carefully but comprehensively.
While age, geography, and comorbidities are major risk factors in contracting or dying from COVID-19, the insights from genetic research are invaluable to effectively tackle this and future pandemics. A growing wealth of data suggest population-level differences — which often do not neatly correlate with popular notions of race — can help us identify susceptibility or resistance to some diseases.
Not exploring this extraordinary phenomenon borders on scientific malpractice, some researchers have told us. By not focusing on the actual science, we are denying the world the fullest understanding of how virus susceptibility works. More importantly, if we followed the ‘race-related’ scientific leads, using the latest genomic data, we could very well come up with measures to better protect most of the rest of the world from COVID-19 and future virus-driven pandemics.
With Africa in particular, the vast genetic diversity within the continent is already revealing much about humanity’s long and deadly evolutionary duel with this scourge. What is more, African researchers themselves are adding to this growing wealth of knowledge, and in some cases taking the lead in population-genetic focused research. This will provide tools to one day eradicate many other diseases that take such a heavy toll on Africa’s peoples.
There are critical hard-wired differences in responses to disease and treatments; some are meaningful. Incorporating them in our health assessments can mean the difference between life and death for the most vulnerable. We ignore them at the peril of the health of billions of people.
Patrick Whittle has a PhD in philosophy and is a New Zealand-based freelance writer with a particular interest in the social and political implications of biological science. Follow him on his website patrickmichaelwhittle.com or on Twitter @WhittlePM
Jon Entine is the founding editor of the Genetic Literacy Project, and winner of 19 major journalism awards. He has written extensively in the popular and academic press on population genetics, including two best-sellers, Taboo: Why Black Athletes Dominate Sports and Why We’re Afraid to Talk About It; and Abraham’s Children: Race, Genetics and the DNA of The Chosen People. Follow him on Twitter @JonEntine