As highly infectious variants of COVID-19 emerge and devastate regions across the world, scientists continue to rely on unexpected foot soldiers for defense: mice. The laboratory mice, unlike humans, are resistant to SARS-CoV-2. Each species is genetically programmed a bit differently. In mice, the ACE2 receptor protein, believed to be the primary mode of entry for SARS-CoV-2 into human cell, is slightly different and does not allow the virus to enter its cells. That makes it an attractive animal to test various hypotheses.
Using genetic engineering technologies, scientists can either introduce human ACE2 gene into mice or convert the mouse Ace2 receptor gene into human type. Such genetically engineered mice serve as valuable models to understand how the SARS-CoV-2 virus, the origin of COVID-19, enters and commandeers a human cell for rapid reproduction.
A mouse containing the human ACE2 gene, was generated some 15 years ago for studying the SARS virus and to find treatments and vaccines against the disease. Because the SARS virus was not causing major problems during the last decade and not many scientists were working on it, the mouse model was put on the shelf—its sperm samples were frozen. When SARS-CoV-2 suddenly became a pandemic disease in the beginning of 2020, the scientific community did not have any animal models suitable for studying it and the cryopreserved mouse model was revived, in a rush in the fall of 2020 by the Jackson Laboratory, a biomedical research institute in Maine that supplies mouse models, useful for various human diseases, to research laboratories around the world.
While this model is useful for general-purpose research and for rapid testing of drugs and vaccines, more versatile mouse models are necessary for finer understanding of the disease process. Such models can be generated using the most popular gene editing tool, CRISPR (Clustered Regularly Interspersed Palindromic Repeats)-Cas9.
In a recent article published in Nature Protocols, genetic blueprints of several types of genetically engineered models were presented that could be used for understanding the molecular mechanisms of SARS-CoV-2 infection and ultimately to test therapeutics and vaccines. The article from the two groups of mouse genetic engineers headed by Dr. Channabasavaiah B. Gurumurthy, University of Nebraska Medical Center, and Dr. Masato Ohtsuka, Tokai University, Japan proposed more than two dozen different types of genetically engineered mouse models suitable for COVID 19 research. Some of these models are specifically designed for studying certain disease conditions. For instance, the designer mice are tailored to exhibit COVID-19 disease in different parts of the body by conditionally expressing ACE2 protein in certain areas.
The proposed models allow researchers to simulate additional disease conditions that occur in human patients, for example, immunocompromised conditions, diabetes or heart diseases. These controlled experiments can be achieved by expressing human ACE2 in only certain tissues in mice or at any given time point in age (young or adult mice).
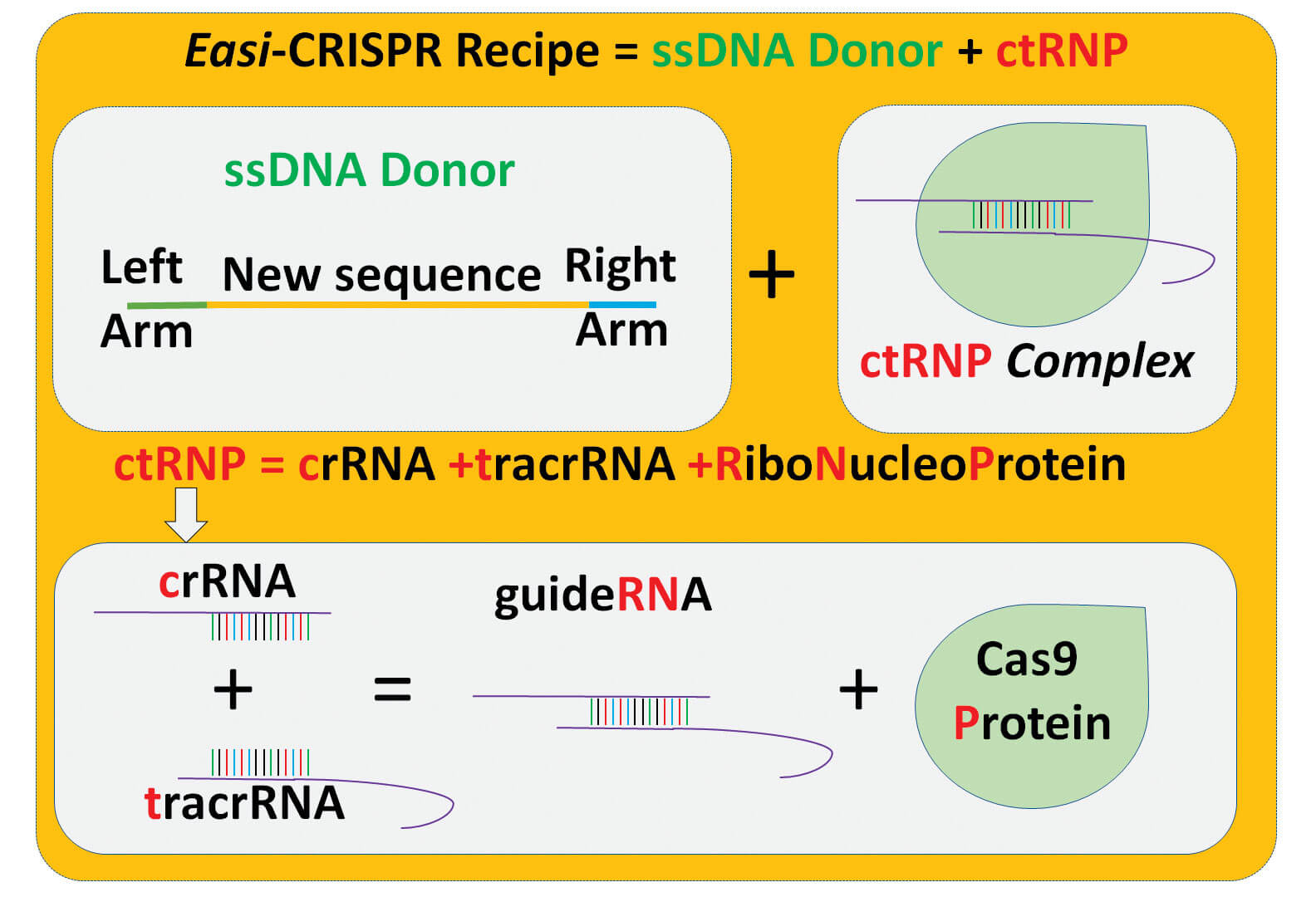
Most of the proposed genetically engineered COVID-19 mouse models can be developed using Easi-CRISPR or i–GONAD technologies that were previously developed in the laboratories of Drs. Gurumurthy and Ohtsuka. Easi-CRISPR method is an efficient version of the revolutionary gene editing technique, CRISPR. i–GONAD (improved Genome editing via Oviductal Nucleic Acids Delivery) is an improvement of CRISPR technology that bypasses several of the complicated steps of traditional genetic engineering technologies.
The protocols of Easi-CRISPR and i–GONAD were published previously in the Nature Protocols journal. The authors proposed genetic blueprints for COVID-19 mouse models using three types of gene editing techniques: knocking out genes, inserting genes and re-engineering previously developed models. These sets of mouse models, once generated, can be bred with various other mice previously developed for other human disease conditions such as obesity, autoimmune disease, cardiovascular disease, hypertension and arrhythmia, ultimately helping scientists to understand the molecular mechanisms of the disease.
Such knowledge will be crucial for developing therapies and vaccines. At present some of the models proposed in the Nature Protocols study have already been developed; the authors are in the process of transferring those mice to Jackson laboratories for expansion of the mouse colonies and for their rapid distribution to scientists around the world working on SARS-CoV-2 and COVID-19 research.
Animal models have advanced our understanding of several of viral diseases like HIV, influenza, measles, herpes and for developing and testing therapies and vaccines against those diseases. They have also been crucial in understanding the molecular mechanisms of the diseases. Even today, several scientists worldwide are still using the models generated many years ago for deeper understanding of the molecular mechanisms of those diseases and to improve therapies and vaccines.
Unlike the well-studied diseases such as HIV, influenza, measles, herpes, that are known to mankind for decades, COVID-19 is relatively a new disease and there is so much we do not know about the virus and the disease. The COVID-19 mouse models will serve as invaluable tools for several decades.
Channabasavaiah B. Gurumurthy has a DVM and a PhD in Virology. He develops genetic engineering technologies and mouse models used in biomedical research. He is Director of Mouse Genome Engineering Core Facility and professor of Pharmacology and Experimental Neuroscience at the University of Nebraska Medical Center. Follow him at his website or Twitter @CRISPRGuru
Masato Ohtsuka has a PhD in developmental genetics. He develops novel technologies of genetic engineering in mice. He is a professor of Molecular Life Science and Molecular Medicine departments at Tokai University School of Medicine, Kanagawa, Japan. Follow him at his website or Twitter @Masato_Ohtsuka
































