The Greek Ministry of Health has issued a stark warning: rapidly multiplying, stealthily infiltrating and carrying a deadly payload of dengue fever, Zika and Chikungunya, mosquitoes and other vector-carrying insects are swarming the country. Their arrival in such large numbers has come as a surprise to health officials, as mosquito-borne diseases are most prevalent in sub-Saharan Africa.
Malaria alone kills nearly one African child under the age of 5 almost every minute, says UNICEF. 2022 saw 247 million malaria cases and 619,000 documented deaths. The disease can cause severe headaches, a shortage of oxygen to the brain, low blood sugar, enlarged liver and spleen, and renal failure. Nine out of 10 of the world’s one million malaria-caused deaths occur in sub-Saharan Africa.
Why the sudden increase in mosquito-borne diseases and deaths? There is a scientific consensus that the increase in the number of disease-vectoring pests is driven by four major factors: changes in land use, water storage practices during droughts, and unpredictable weather patterns as the climate warms.
Global NGOs and government agencies including the Intergovernmental Panel on Climate Change (IPCC), Centers for Disease Control, the EPA, NASA and the United Nations’ World Health Organization all cite evidence of warming temperatures which increase insect mobility.
WHO believes the ease by which mosquitoes and other pests can move across continents puts many regions in peril. According to Raman Velayudhan who heads the agency’s global program on the control of neglected tropical diseases:
[C]limate change has played a key role in facilitating the spread of the vector mosquitoes down south. And then when people travel, naturally the virus goes along with them. And this trend is likely to continue for the rest of the world.
Climate change has resulted in an increase in droughts, heatwaves, floods, and rainfall, says Dr. Katie Anders, an epidemiologist and director of impact assessment at the World Mosquito Program (WMP). It also has increased mosquito-borne disease risk in less obvious ways:
For example, when households store water in response to drought, this can increase local mosquito breeding sites and disease risk. Land use changes can also drive migration to cities, increasing the population at risk of explosive outbreaks of dengue and other mosquito-borne diseases.
But that might only limit the problem, not contain it. Lowering global temperatures is a multi-decade endeavor, assuming it’s possible, and the impact of lowering carbon emissions on decreasing malaria would be minimal. Even if carbon limits are put in place and they work beyond expectations tens of millions of lives — perhaps in the billions — could be lost in vulnerable countries.
And identifying a driving cause of a problem does not suggest an easy solution. Bjorn Lomborg’s controversial Copenhagen Consensus Center estimates spending tens of trillions of dollars a year on reducing carbon might reduce the at-risk malaria population by only 3%.
There is one solution embraced by global health experts that should be pursued aggressively, if with some caution. Scientists in real-world trials have altered the genomes of entire animal populations, including mosquitoes, to thwart the vectoring of diseases and control pests — an innovation called gene drives. Emerging gene drive technologies offer enormous potential and have already shown their value in test projects in many parts of the world. More recently, the application of CRISPR/Cas9 tools has dramatically accelerated their effectiveness.
But implementation on a wider scale is progressing at a snail’s pace. Why? For the most part, it is restrained by controversy, misunderstanding and the political opposition of activist environmental groups in Europe and North America.
New wave of more destructive disease-carrying mosquitoes
The resurgence of mosquito-borne diseases and the persistence of auto-immune diseases such as Lyme disease have set alarm bells; none of the proposed solutions attacks the root of the problem: the mosquitoes themselves.
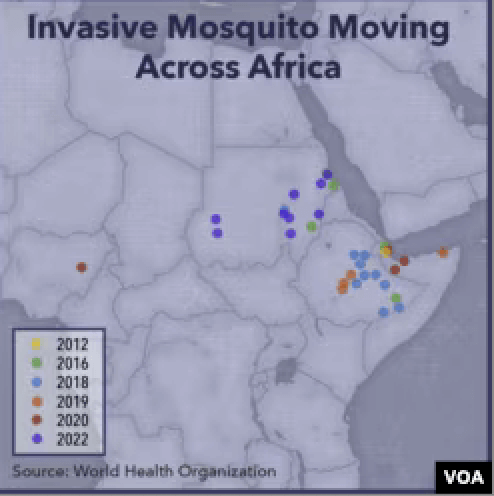
Eastern Africa has been particularly hard hit in recent years with the arrival of a species new to the region. “[A]n invasive species of mosquito … has threatened to unravel two decades of gains in malaria control” in Ethiopia, noted the Voice of America.
What made this spike in cases unusual is that it happened outside the rainy season, when malaria typically surges across Africa…. Cities are not immune, but they typically don’t see these kinds of outbreaks.
Anopheles stephensi was previously known to spread malaria only in South Asia and parts of the Arabian Peninsula. This species has exacerbated a confluence of challenges: it is adept at evading available insecticides and vectoring disease; it’s almost undetectable; and “you can’t kill it with a drug,” said Fitsum Girma Tadesse, a molecular biologist at Ethiopia’s Armauer Hansen Research Institute.
This past spring, it spread across Ghana and Kenya. This is a sharp turnaround from a decade ago when progress was being made in controlling pest-vectored diseases. Deaths from malaria had plunged from nearly one million at the turn of the century to 560,000 in 2015. Communicable disease experts are worried we may be in the early stages of an escalating global scourge that gathers storm in Africa before spreading globally.
“Saying the world is getting hotter is just the start of the story”, says Professor Laurie Zoloth of the University of Chicago’s MacLean Center for Biomedical Ethics at the Pritzker School of Medicine. Zoloth has written several books about the interplay between environment, socioeconomics and disease spread. Her upcoming book, “May We Make the World? Gene Drives, Malaria, and the Future of Nature”, addresses this tension point.
Globalization has set the world up for vector-borne diseases. Public health interventions are powerful, but this has always lagged behind for the poor. Climate change contributes but our neglect of the communities in places like Africa promotes the spread.
While climate change is exacerbating the health challenges of course, but a growing number of experts believe that focusing primarily on carbon reduction as a solution to malaria could cost hundreds of millions if not billions of lives.
We have gene drive tools to address this scourge, Zoloth says, but they are not being utilized, mostly because of ideological concerns.
Containing disease vectoring
Trying to control mosquito-borne diseases is not a new challenge. Developed in the 1940s, dichlorodiphenyltrichloroethane — DDT — became widely used to combat malaria, typhus and other insect-borne diseases among the civilian and military populations. Its effectiveness led to significant declines in malaria cases in many regions. However, concerns about its environmental impact, including its persistence in ecosystems and potential harm to wildlife and human health, has led to its restriction.
Claims by some environmental groups that it is unsafe and banned are exaggerations with some truth. The US banned DDT except for emergency use in 1972 in the midst of the controversy stirred by Rachel Carlson’s “Silent Spring”, which raised concerns about its impact on wildlife. Many scientists, then and now, challenged the ban. With backing by WHO, many developing countries still promote its selective use. Even the New York Times has endorsed the pesticide as one of the only effective tools to contain malaria.
Some experts believe that if DDT and other insecticides were made available for use in the developing world after the Silent Spring controversy, we may not be facing the current crisis.
Lomborg advocates funding the production and distribution of insecticide-treated bednets, which prevent bites, kills mosquitoes and disrupts transmission. He estimates that could reduce malaria deaths to less than 300,000 annually at a yearly cost of a little more than one billion dollars. But many environmental advocacy groups opposed to using insect-repelling chemicals despite their known effectiveness.
While chemical-based approaches, old and new, offer some value, at best they could reduce incidences of insect-borne diseases but not eliminate them. That’s why increasing attention is focused on gene drives.
“We might have been able to wipe out malaria with DDT, but we stopped before the poorest countries got it,” Professor Zoloth says. “We have an obligation to end human suffering. Something new needs to happen. We have been using 19th-century tools and need a better solution”.
Could gene drives help contain disease-vectoring?
The advent of CRISPR gene editing opened the door for scientists to alter how genes in mosquitoes are inherited the population. Gene drives are genetically engineered to “drive” a desired gene throughout a procreating population by creating so-called “selfish genes”. These gene drives accelerate the spread of an altered gene through subsequent generations. This drive leads to the suppression or alteration of certain deleterious traits or can even eliminate a targeted population — like certain disease-vectoring mosquito species.
Here’s how gene drives work in mosquitoes. Only female mosquitoes bite and pass on diseases like malaria. Scientists have devised ways to ensure that propagating females either die or are effectively sterile, so they cannot spread a virus. One technique, developed with funding from the Gates Foundation, introduces a gene drive in males that pass on a mutation to females that deforms their mouth, making them unable to bite and spread the parasite. It also deforms their reproductive organs so they cannot lay eggs.
Other gene-editing techniques to control disease vectoring are in development. In laboratory settings, malaria-carrying mosquitoes have been tweaked using CRISPR in a way that slows the development of malaria parasites inside them and also reduces the lifespan of the mosquitoes. The result is that the modified insects die before they can spread the disease.
Proof-of-concept for the effectiveness of gene drives is planned. The Gates Foundation has funded a successful trial release of non-gene drive genetically sterile mosquitoes in Burkina Faso in 2019. Researchers in Burkina Faso and Ghana are also designing field trials of genetically modified self-limiting mosquitoes on a phased pathway to potential gene drive field trials in the future.
The UK-based biotech company Oxitec, in cooperation with the Brazilian government, has successfully released two different types of laboratory-altered mosquitoes in two different locations and at scale. These were not produced using CRISPR. Rather, the mosquitoes were engineered to produce sterile male offspring when they mate with females with the aim of reducing the population of disease-carrying Aedes aegypti mosquitoes. Oxitec has also conducted successful trials of this mosquito breed in Brazil and the Florida Keys. Its major drawback: the lab-grown sterile mosquitoes need to be released numerous times to control the disease-carrying ones.
It’s also eager to test-run a new version of its technology, in California’s changing climate. Oxitec recently received approval from the EPA to release genetically modified male mosquitoes in the Central Valley. In this case, Oxitec genetically engineered sterile male mosquitoes that don’t produce female progeny to vector disease. to produce offspring that don’t survive. Hence there are no new generations of females to pass along disease.
Another company, MosquitoMate, has developed a non-CRISPR gene drive, conducting trials in Fresno, CA and West Keys, FL stirring almost no public attention. It’s approved for use and available for home and institutional use.
Controversy and critics
Mustapha Debboun, general manager of the Delta Mosquito and Vector Control District in Visalia labelled gene drives as “ingenious”. “Instead of using a human being to apply a pesticide to kill these mosquitoes, we’re using male mosquitoes to do the job for us …. It’s nature against nature.”
“In my opinion, the use of gene-drive mosquitoes are going to be effective against the spread of malaria or other vector-borne diseases,” said Jeantine Lunshof, a bioethicist at the Wyss Institute for Biologically Inspired Engineering at Harvard University.
Bioethicist Zoloth agrees, and not only because of its scientific effectiveness. “I like gene drives because they can inhibit mosquito competency. Gene drives are also capable of changing life circumstances for the poor. They aren’t set up to make someone a lot of money and they don’t cost a lot of money,” she told the GLP.
Gene drives are still in the early stage of development so caution is appropriate. How do you develop a system that by its very nature requires so few individuals to initiate the irreversible invasion of an entire target population? This Catch-22 is the basis of the objections voiced by anti-biotechnology NGOs who warn of a global environmental crisis if gene drives are allowed to be introduced. Critics have called gene drives a “genetic atom bomb,” claiming if a gene drive spread beyond its target population there would be no way of stopping it.
They have a point. There is a chance, though very small say experts in the field, that altering a species using a gene drive could lead to ecological complications. In an extreme, worst-case scenario, gene drive that would eradicate an entire mosquito population in a given area could disrupt the ecosystem in unforeseen ways, such as ‘jumping’ into a non-target species, including humans.
But natural gene drives exist in nature with no consequences. And that’s why most of the eradication projects are pilot studies. Scientists do believe that gene drive “off switches”, in development, could render this concern mute.
As Vox has pointed out, this remote potential for harm needs to be balanced against the actual risk of not utilizing a life-saving innovation. Disease-carrying mosquitoes kill between 1,200 and 2,000 people, mostly children, every day.
But many environmental NGOs, which reject genetic modification in agriculture as well, cite the precautionary principle, express concerns about potential consequences and the lack of transparency in the project. According to Friends of the Earth program manager Dana Perls claims there is always a possibility of unintended consequences and what they believes is a lack of transparency:
The science was incomplete before the first rollout. There’s no such thing as 100 percent effective in science. Yet the public is being asked to trust that Oxitec’s experiment will work and no [genetically-engineered] female mosquitoes will survive. But how do we know that?
Other critics sat gene drives are inherently too dangerous, citing versions of the popular (but unscientific) ‘butterfly effect’.
“The idea of gene-drive mosquitoes is something that is very disturbing to me and to many of the people I speak to,” said Nnimmo Bassey, who heads the Health of Mother Earth Foundation in Nigeria, an environmental advocacy group. “It has the possibility of disrupting the balance in our ecosystems” in ways that can’t be predicted.
Additionally, critics have expressed concerns regarding the potential interaction between the modified mosquitoes and Tetracycline, an agricultural antibiotic present in wastewater. They worry that this interaction could create a complex situation where female mosquitoes develop and result in the emergence of hybrid mosquitoes that are harder to manage or control.
Looking to the future, the battle against mosquitoes and disease vectors requires a comprehensive understanding of the complex factors at play. Gene drives offer a promising avenue for managing mosquito-borne diseases by altering mosquito populations and reducing disease transmission. Proof-of-concept trials, such as the Oxitec, MosquitoMate and Gates-funded research, provide a glimpse into the potential of this fast-evolving technology.
Public health officials need to vociferously address reasonable concerns with evidence-based information to ensure the gene-drive strategy is not lost to alarmist propaganda. The CDC could also do well to update its “Integrated Mosquito Management” strategy. It outlines a variety of vector control measures, including the use of insecticides, mosquito nets, and the elimination of breeding sites, that have successfully reduced disease transmission. There is no mention of the most promising strategy to date: gene drives.
Sam Moxon has a PhD in tissue engineering and is currently a research fellow in the field of regenerative medicine. He is a freelance writer with an interest in the development of new technologies to enhance medical therapies. Follow him on Twitter @DrSamMoxon