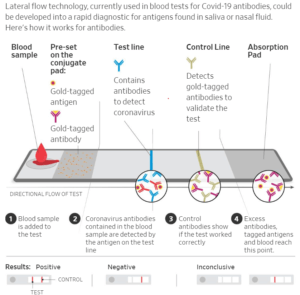
To stretch beyond the lab, test developers are racing to produce next-stage technologies that could allow for rapid widespread testing as quickly as an at-home pregnancy test.
…
[T]he National Institutes of Health announced a competition meant to speed up development of diagnostic technologies, with the goal of millions of rapid tests a week available by the end of summer, and more by flu season. The Rapid Acceleration of Diagnostics, or RADx, initiative, often compared with the TV show “Shark Tank,” will provide finalists with up to $500 million and technical, business and manufacturing expertise. Over 1,700 groups have registered, more than 280 have applied, and 40 have advanced to a “deep dive” review stage.…
Whether the diagnostics industry can ramp up to produce millions of rapid tests a week by the fall remains an open question. The NIH’s Dr. Tromberg and others say it can be done. OraSure said it aims to submit its at-home test for FDA authorization in September, while Alveo Technologies and others anticipate seeking authorization in late 2020 or 2021.