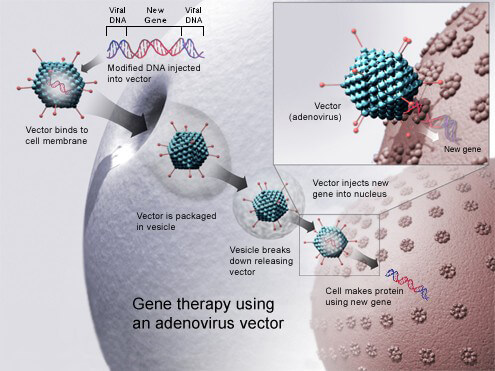
Gene Therapy

A golden age of gene therapy is now in sight
To date, just two in vivo gene therapies have been approved in the United States. Both are for what [Duke ...

New hope for Duchenne muscular dystrophy (DMD) patients? CRISPR cured a mouse — and humans could be next
DMD is a fatal — and currently incurable — genetic condition that causes the body's muscles to deteriorate over time. ...
Cure for sickle cell diseases inches closer with launch of gene therapy trial
For 65 years, scientists have known the cause of sickle cell disease but have been unable to cure it without ...

Gene transfer therapies are the ‘next big thing’ in medicine. Here’s how gene editing works and the companies behind it
The breakthroughs made possible by gene editing were shown in the Jan. 6 news that base editing had repaired a ...

Delay aging and extend our lifespans? Gene therapy might be able to do that
How many aging-promoting genes are there in the human genome? What are the molecular mechanisms by which these genes regulate ...
30+ genes identified that could protect us from COVID, opening door to gene therapy prevention solutions
The goal was two-fold: to identify the genes that make human cells more resistant to SARS-CoV-2 virus; and test existing ...
Gene therapy may be able to restore vision
In a normal eye, opsins are expressed by the rod and cone photoreceptors in the retina. When activated by light, ...

Resurgence of gene therapy has dramatically altered the the biomedicine revolution
Some technologies that have emerged and altered the landscape in recent years include immunotherapy, CRISPR-Cas9 gene editing, and chimeric antigen ...

‘Gigantic leaps forward’ What’s happened in the 21 years since a teenager died undergoing gene therapy, stalling emergence of this biomedical breakthrough?
In 1999, [teenager] Jesse [Gelsinger] received a dose of the ornithine transcarbamylase gene, engineered into a recombinant adenovirus, at the ...

Podcast: Rare genetic disorders and pregnancy—Navigating an ’emotionally challenging’ journey
We look at the progress that’s been made in tackling rare genetic disorders (and the challenges that remain) and we ...
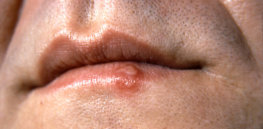
Herpes cure? Gene therapy may work
Infectious disease researchers at Fred Hutchinson Cancer Research Center have used a gene editing approach to remove latent herpes simplex ...
Gene therapy for hemophilia delayed until 2022 after FDA rejects one-time treatment, shocking doctors and scientists
U.S. regulators rejected [Biomarin’s] potentially game-changing hemophilia A gene therapy over concerns it might not really be a one-and-done lifetime ...

One year in, 9 boys with muscular dystrophy show remarkable progress from gene therapy
Nine boys aged 6 to 12 who have been living with [Duchenne Muscular Dystrophy] since birth received a gene therapy ...
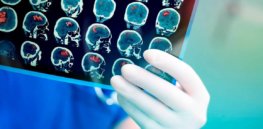
Strokes often deadly but gene therapy offers hope to rebuild critically damaged brain cells
When you think of a typical meal in the US South, you will likely recall the rich, hearty dishes that ...

Lucy may not live to see her second birthday without expensive gene therapy
Lucy Van Doormaal was born on April 1 in Vancouver, but before her parents, Scott and Laura Van Doormaal, could ...

Another COVID-19 casualty: ‘Major delays’ for gene therapy trials for rare diseases
While the ongoing COVID-19 pandemic won’t have much of an impact on cash available for new biotech startups, it has ...

Gene therapy could restore color vision for people who see the world in shades of gray
In a small trial in Germany, an experimental gene therapy improved the vision of nine people with total color blindness, ...

Ultimate fitness hack: Imagine being able to build muscles with a gene therapy
Trying to hack fitness is a multi-million-dollar industry; we’ve all seen at least one ad featuring a purported miracle product ...

What’s holding up gene therapy? Making drugs on a large scale
As interest in gene therapy grows, manufacturers face physical, biological, and engineering challenges to developing and producing drugs on a ...

Podcast: Fighting blindness with CRISPR. Ophthalmologist in groundbreaking study explains how gene editing could treat a once-incurable disease
Congenital eye disorders can rob children of their eyesight at a young age and severely diminish their quality of life ...
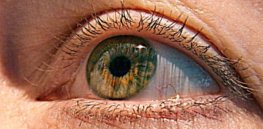
Common form of inherited blindness shows ‘massive improvements’ with experimental gene therapy
This month, K.L. became one of the first patients to receive a new experimental gene therapy for children with a ...

Building ‘better’ astronauts through genetic engineering could be key to colonizing other planets
Through genetic engineering, we will one day have the ability to thrive in harsh alien environments ...

Gene therapy shows success against some cancers and inherited disorders. Can it tackle obesity?
Scientists are working with treatments that have shown success in amping up the metabolism of mice, helping them lose weight ...

900 gene therapy drugs are in the pipeline. How does the FDA want to regulate them?
To date, the FDA has approved four gene therapy products, which insert new genetic material into a patient’s cells. The ...

Gene therapy for Alzheimer’s? Biotech startup suggests new approach, using dementia ‘off switch’
What if there were a single mechanism in the brain that, when faulty, leads to all kinds of dementias? And ...

Teenager’s experimental gene therapy treatment could change the lives of millions of sickle cell patients worldwide
Meet Helen Obando, a shy 16-year-old who likes to dance when her body isn’t ravaged by the debilitating symptoms of ...
Gene therapy era: A look at current treatments and what’s next for cancers and genetic disorders
At least nine gene therapies have been approved for certain kinds of cancer, some viral infections and a few inherited ...