On the surface, this may seem like an inflammatory question, bringing together the loaded term ‘discriminate’ and the contentious and the historically problematic concept of ‘race’—all set against the xenophobic backdrop of the current Covid-19 pandemic. But it’s precisely the ballooning coronavirus crisis that suggests the answer might be in part, ‘yes’.
And if that’s the case, what can we learn as scientists around the world rush to unmask the genetic mysteries of the spreading pandemic and come up with viable treatments or a vaccine that works for as many people as possible? This is a question that must be carefully asked and precisely answered.
A review of the world maps of confirmed cases of the coronavirus and the deaths that it has caused shows an unusual anomaly: The world’s second most populated continent, Africa, has seen the fewest number of confirmed cases per capita, 7080, through April 3, despite the fact that Africa has extensive trade and travel relations with China, where the outbreak originated, and with Europe, a global hotspot.
The most affected area extends across North Africa and Northeast Africa, with Morocco, Algeria, Tunisia, and Egypt accounting for about half of the continent’s reported cases. 50 of Africa’s 54 countries have recorded cases (all excerpt Comoros, Lesotho, Sao Tome and Principe and South Sudan). Based on reported cases, sub-Saharan Africa has been spared much of the scourge except for multi-racial South Africa, the continent’s warm spot, which has recorded the most cases on the continent with 11462, and ranks 44th in the world. 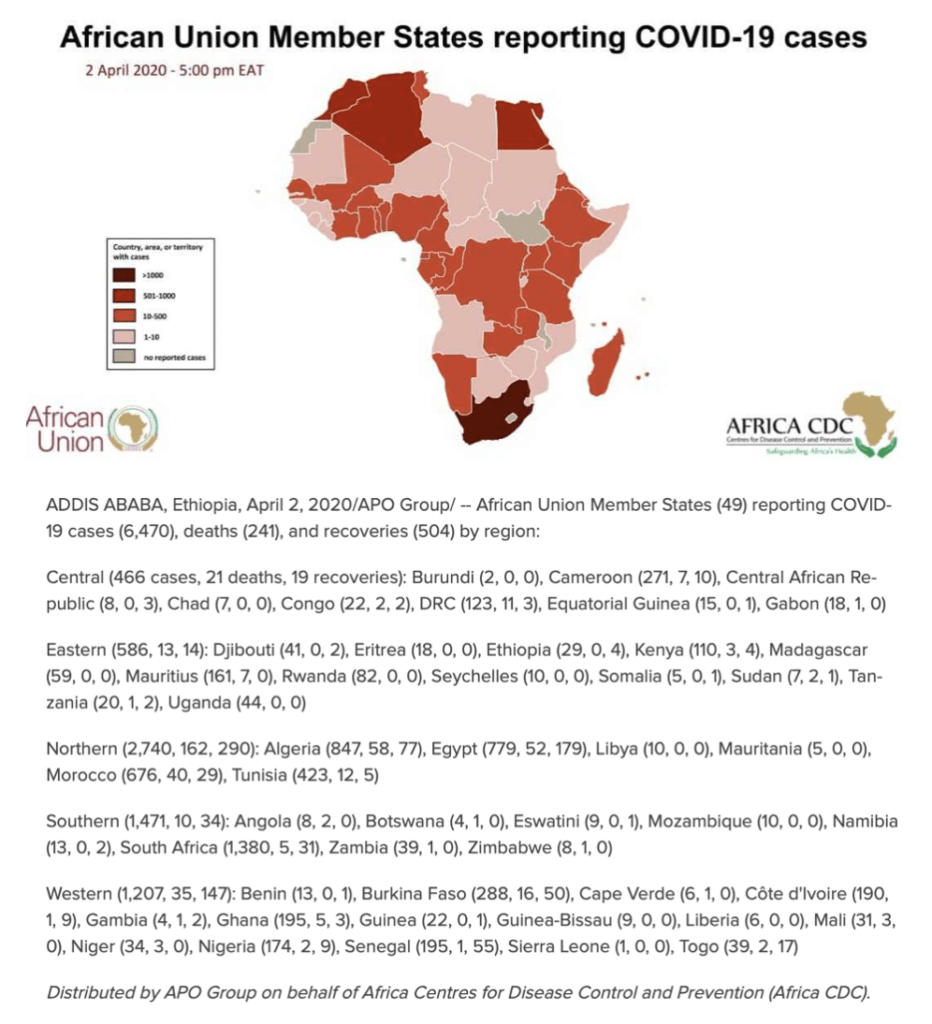
The number of death per capita in sub-Saharan Africa is among the lowest in the world, less than 300 as of April 5, a fraction of that reported in most countries (NOTE: The infrastructure to report cases and deaths is not robust in many African countries]. [Our World in Data] Even in South Africa, the death toll per million people is far lower than the numbers reported on other continents.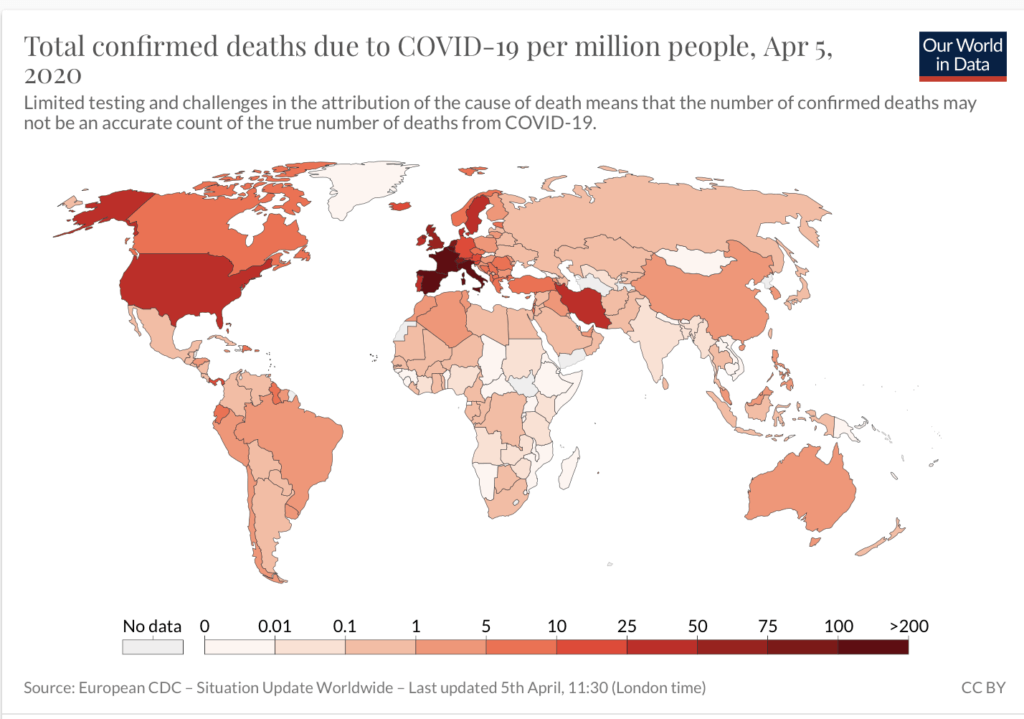
Environmental factors
Before we begin exploring potential genetic explanations for this phenomenon, it’s important to address the environmental and cultural explanations for disease prevalence in particular populations. The apparent low incidence of Covid-19 in Africa could be due to under-reporting or limited testing (although major outbreaks, akin to those in South Korea, Iran or Italy, would likely be evident by now).
 Burundi is one of the few African countries without any confirmed cases of the virus. When the nation’s Minister of Health was asked about the secret behind the zero number of Covid-19 cases, he said: “It is very simple. We don’t have the testing kits.” According to the WHO, “most” of the countries in Africa with testing capacity only have between 100 and 200 testing kits. So as many of the countries in Africa have done fewer tests that other countries in other regions, the numbers may not adequately reflect what could be a larger infected population.
Burundi is one of the few African countries without any confirmed cases of the virus. When the nation’s Minister of Health was asked about the secret behind the zero number of Covid-19 cases, he said: “It is very simple. We don’t have the testing kits.” According to the WHO, “most” of the countries in Africa with testing capacity only have between 100 and 200 testing kits. So as many of the countries in Africa have done fewer tests that other countries in other regions, the numbers may not adequately reflect what could be a larger infected population.
Another factor could be the relatively youth of Africans. While the median population age in Eurasia and North America is nearer 40-years old, in Africa it is below 20 (for example, the median age in the UK is 40.2 and in China 37; by contrast, it is 17.9 in Nigeria.) There is accumulating evidence that the young, when infected, are less apt to show symptoms, and asymptomatic people are not being tested, perhaps suppressing the numbers. In addition, while some cities, such as Lagos, are among the most densely populated in the world, large parts of Africa are not as dense, and this, along with the continent’s fragmented transport networks, may hinder virus transmission. But even granting those differences, the disparity in cases and deaths among Africans as compared to the rest of the world is striking.
In one of many possible comparisons, according to Worldometer, as of April 3, Nigeria, whose capital Lagos is one of the largest and densest population centers in the world, and which has one of the best health care systems in Africa, ranks 101st in number of cases globally and has 190 reported cases and 2 deaths, a mortality rate of 1.1%; Italy, which has the fourth highest number of cases in the world, and with its far-superior medical facilities and testing capacity, there have been 115,242 cases and 13,915; a mortality rate of 12.1%.
Considering the pattern emerging that the biggest threats to Africa are from foreigners entering the continent, many African countries are shutting their doors to visitors from Europe, Asia and North America to prevent the possible spread of the virus — 32 countries in all. Even before Djibouti recorded a single case (it now has 49), it suspended all international flights to the country, according to the US Embassy in Djibouti. This emerging global anomaly is beginning to attract the attention of researchers who are looking for genetic factors that might provide clues to the viruses’ origins and vulnerabilities.
‘Race’ vs. population
Despite the fact that the social conception of ‘race’ does not neatly mirror human biological differences, could genetic factors based on population differences thousands of years in the making account for some of the difference in health outcomes between different human groups?
The question is not as inflammatory as it may seem in our politically sensitive world. Given a broad interpretation of the word ‘population’ as simply those who share features in common, there are clear differences between specific populations: The sub-population most at risk from Covid-19, for instance, is the elderly, with age-related deterioration of the immune system the most obvious biological explanation for this group’s higher rate of mortality.
What, though, of racial populations? Again, we need not jump only to a biologically based answer. Racially defined populations could be at greater risk, but not necessarily for directly biological reasons. Those who are poor or in poor health are at greatest risk. Given the clear inequalities faced by many minority peoples, social and environmental factors–deprivation, marginalization, inadequate access to health care, and the like–is powerful evidence of the heightened vulnerability of many racial populations. Thus ‘race’ (defined as a social not a biological concept) could indeed have a bearing on the severity of Covid-19, most especially in societies where racial inequalities are deep and persistent.
So let’s rephrase our initial question: Could some ‘populations’ more or less susceptible to Covid-19 because of their evolutionary history?
Given the odious racist history of biological beliefs about human differences, this is a fraught question to ask, let alone attempt to answer. Social scientists, for example, emphasize the socially constructed nature of ‘race’, while rejecting as superficial the possible biological basis to observable differences between racial populations. Population geneticists also acknowledge the myriad social factors that lace through the historical concept of race, and its frequent misuse to support odious racial hierarchies; nevertheless, they also highlight the growing wealth of data that suggests that individuals in pockets of populations, some small and large, are more likely to have certain genotypically based phenotoypes across certain characteristics. Scientists don’t refer to these as ‘racial differences’ but rather call them population-based differences, and they don’t always correlate with popular and often problematic notions of race.
Although geneticists and the general population generally avoid using the term “racial” to characterize disease proclivities that show up more in one population than others, ancestry matters. Is there something about Africa’s population as a whole that might explain this (seeming) reduced susceptibility? Or, to cut to the chase, could that ‘something’ be genetic differences among African and non-African populations?
The history of another horrendous global pandemic, that of HIV/AIDS, suggests why this is an issue worth pursuing, even if this inquiry makes some uncomfortable and caution must be taken. In contrast to the apparent situation with Covid-19, sub-Saharan Africa was (and remains) more seriously afflicted by the scourge of HIV than any other region of the world. (And while HIV has killed millions of people in Africa, and continues to kill hundreds of thousands each year, this receives little attention in the rest of the world currently fixated on the Coronavirus.)
Importantly, genetic factors have long been implicated in the apparent increased vulnerability of African populations to HIV (and other recently introduced diseases such as tuberculosis). Around 11% of HIV infections in Africa may be due to a genetic variant common in people of African descent that makes them more vulnerable to the virus. The genetic change, which is less prevalent in other ethnic groups, increases the likelihood of infection with the most common strain of the virus (HIV-1) by 40%.
If some genetic variants potentially play a part in a population’s increased susceptibility to certain diseases (such as HIV), then it is prudent to ask whether other variants could act as a prophylaxis for other diseases (such as Covid-19). And in either case, any increase in our understanding of the genetics of disease resistance can only aid our efforts to develop treatments for those most at risk. Environmental and genetic influences on disease susceptibility are not mutually exclusive. In the case of HIV, for example, possible genetic influences merely compound the effects of poverty and inadequate medical care among the most vulnerable groups. In addition, there is also clear historical evidence of population differences in immunity to contagious diseases such as Covid-19.
Evolution and disease
History provides some context to this discussion. Recent genetic evidence suggests that pestilence played a key part in the displacement of Europe’s original Neolithic population by genetically dissimilar peoples from the east, around 5,000-6000 years ago. Here, the newcomers’ longer exposure to the plague bacterium, Yersinia pestis, likely provided the protection of partial immunity, a genetic advantage not shared by the existing human populations.
A similar process, though on a far greater scale, followed the European expansion around the globe from the 1400s onwards. Biogeographer Jared Diamond, for instance, estimated that over 90 percent of the indigenous peoples of the Americas—never before exposed to the deadly contagions carried by the foreign explorers and colonists—were wiped out within decades of the arrival of Europeans. And again, evolved immunity to the diseases that had plagued Eurasia for millennia gave the invaders a genetic advantage over the established populations.
In his highly influential Guns, Germs & Steel, Diamond argues that susceptibility to disease was one of the main causes of the racial inequalities that are clearly evident even today. But of most relevance here is Diamond’s account of the ancient origins of these diseases—an account that matches the modern emergence and spread of SARS-COV-2 (the virus that causes Covid-19).
After the advent of agriculture in Eurasia (c. 10,000 years ago), farming peoples’ close contact with domesticated animals allowed novel infections to leap from animals to human beings, with these societies’ denser populations then providing the breeding grounds within which these diseases could further mutate and spread. Over time, the deadly contagions that periodically swept along Eurasia’s trade routes then genetically selected for disease-resistance among the surviving populations.
And SARS-COV-2 has indeed followed a similar trajectory (as did the related coronavirus behind the 2002 SARS epidemic), first crossing the species barrier in an crowded animal market in a densely populated city in China, before rapidly spreading along our modern transport equivalents of the ancient trade routes.
Of course, the sudden recent emergence of Covid-19 means there has been no time for the additional aspect of Diamond’s thesis–the natural selection of disease resistance within affected communities. The important point, though, is that the different impact of disease on different human populations has long been a feature of our species’ history—and, moreover, that these different outcomes can be explained in genetic terms (in addition to the social factors raised above).
And this brings us back to the possibility of some form of genetically mediated immunity to Covid-19 in modern African populations. If there is indeed a basis to this speculation, any such immunity cannot have been naturally selected–it must instead be a random consequence of other genetic variation that exists within the wider African population. And again, HIV provides a good example–especially as the genetic variants that increase susceptibility to the HIV virus among modern sub-Saharan Africans are believed to be the result of natural selection for genes that reduced vulnerability to malaria in an ancestral population. By extension, different genetic variants, selected for different reasons in ancestral African populations, could possibly account for the apparent reduced vulnerability to Covid-19.
Importantly, this could also be true for any human population, African or non-African, that appears to show a different response to diseases like Covid-19. Potentially, this is the consequence of natural selection for particular genes in an ancestral population of that specific human group.
Yet there is an important reason why African populations are more likely to be a source of any such genetic variants–namely, because Africa, as the ancestral home of our species, contains more human genetic diversity than anywhere else on the planet, and also carries genes from ancient ‘ghost populations’. Or, put another way, the genetic variety of all non-African human populations is a mere subset of the wider diversity evidenced in today’s African populations.

According to the prevailing ‘Out of Africa’ theory, the major expansion of modern human beings across the globe occurred within the last 100,000 years. And recent genetic research demonstrates that these human populations encountered and interbred with other closely related human groups, acquiring advantageous genes as a result (e.g., genetic defences against viral infections picked up from Neanderthals, or the ability to cope with high altitudes acquired from Denisovans). And yet this has only occurred within the last tens of thousands of years–by contrast, humans and their close relatives have existed in Africa for millions of years.
Given the long research bias towards Eurasia, it is only now that modern techniques for probing ancient DNA are revealing the different migrations of different ancestral human groups that occurred across Africa across this huge amount of time. And, as was the case on a much smaller scale in Eurasia, these ancestral populations undoubtedly acquired numerous locally advantageous genes—including genetic defences against diseases—either directly through natural selection or indirectly through intermingling with longer established groups. (For example, recent studies suggest that some modern West Africans, such as these Mende people in Sierra Leone, carry a small but helpful genetic inheritance from a previously unknown, now-extinct hominid population.)
What about disease and ‘race’?
Is it therefore suspect—even racist—to explore the relationship of disease to population? Although the historically loaded term of ‘race’ is considered a controversial subject, geneticists recognize genetic differences among population groups that evidence themselves in disease proclivities. Renowned population geneticist Neil Risch and colleagues addressed this issue years ago in what’s considered a seminal paper, “Categorization of Humans in Bio-Medical Research: Genes, Race and Disease, writing:
… we believe that identifying genetic differences between races and ethnic groups, be they for random genetic markers, genes that lead to disease susceptibility or variation in drug response, is scientifically appropriate. … Every race and even ethnic group within the races has its own collection of clinical priorities based on differing prevalence of diseases.
In some cases, a person’s ancestry can affect susceptibility to a disease or response to treatment. Certain disorders are more prevalent in population groups that were relatively insular over centuries, staying in culturally or geographic pockets and intermarrying (e.g. Basques, Icelanders, Ashkenazi Jews, Amish, West Africans). As a result, there are thousands of genetic disorders, hundreds of which disproportionately affect one group more than another. Although geneticists and the general population generally avoid using the term “racial” to characterize disease proclivities that show up more in one population than others, ancestry matters.
There are numerous genetic diseases that impact those of sub-Saharan African ancestry disproportionately as a result of their evolutionary history, most notably sickle cell anemia, which affects certain populations in Africa, Arabia and the Mediterranean. Those of African descent are more susceptible to heart disease and are 50 percent more likely than those of European descent to die of colorectal cancer, even if they receive the same treatment. The variant of one gene may explain why women of African descent have twice the risk of premature delivery than those of European ancestry. Some two million people of European ancestry worldwide now carry copies of a mutant gene that makes them immune to HIV while some sub-Saharan population groups seem more susceptible to it. On the other hand, although rare in people of African ancestry and Asians, cystic fibrosis and multiple sclerosis are lethal genetic diseases in those of Northern European ancestry.
There is a danger in over-simplifying and ‘racializing’ this data. The notion of ‘race’ and the historical prejudices attached to that socially-constructed notion are well known and odious. Both for genetic and non-genetic reasons, we believe that racial and ethnic groups, which are often not definable populations, should not automatically be assumed to be different or equivalent in terms of disease risk or drug response or any phenotype. But ‘race’ can serve as a loose marker that can inform research. A ‘race-neutral’ or ‘color-blind’ approach to biomedical research poses its own dangers, and may prove neither equitable nor advantageous, and would not necessarily lead to a reduction of disparities in disease risk or treatment efficacy between groups.This perspective—mainstream in the genetics community—was reaffirmed two years ago in an New York Times’ essay by Harvard University David Reich, writing:
Recent genetic studies have demonstrated differences across populations not just in the genetic determinants of simple traits such as skin color, but also in more complex traits like bodily dimensions and susceptibility to diseases. For example, we now know that genetic factors help explain why northern Europeans are taller on average than southern Europeans, why multiple sclerosis is more common in European-Americans than in African-Americans, and why the reverse is true for end-stage kidney disease.
I am worried that well-meaning people who deny the possibility of substantial biological differences among human populations are digging themselves into an indefensible position, one that will not survive the onslaught of science.
Whether those defined as African Americans, Hispanics, Native Americans, Pacific Islanders or Asians have natural antibodies to a disease or respond equally to a particular drug is an empirical question that can only be addressed by studying these groups. There is some very weak, anecdotal evidence. There are reports that no Nigerians were infected in China even though there are many, particularly in Wuhan, because of those countries close trade relationship. There is also some speculation that there may be resistance carried by people with the common African blood group O. But no formal studies of this kind has been done so far on the Covid-19 coronavirus. Omolade Awodu, a professor of haematology at the school of medicine at Nigeria’s University of Benin, was asked whether African blood and black skin made people resistant to coronavirus. “This is a new virus and very little is known about it,” she said. “However, I have not come across any research that confirms the claim that African blood composition or black skin resist coronavirus.”
What genetic factors could be at work in Africa impacting infections and deaths?
Research and informed speculation is already underway. There has been some speculation that blood type O could be associated with resistance. Blood type O is highest among Africans and those of African descent. It is carried by 51% of African Americans, compared to 45% of whites, and 40% of Asians. Blood type A, which is associated with Covid-19 susceptibility, is carried by 26% of African Americans and 40% of whites. There is some evidence that those with Blood Type A are more susceptible to COVID, whereas those with O are less so.
Susceptibility to Covid-19 has been found to be associated with a high expression of the ACE2 receptor gene in the lungs. A recent study has found that Africans are considerably lower than East Asians in the expression of ACE2 receptors, though slightly higher than Europeans.
Since this article was first posted, the University of Hawai’i Cancer Center announced it was in fact pursuing the very issue of racial and ethnic disparities in the susceptibility of Covid-19, focusing on genetic variants of the ACE2 gene. “Epidemiological studies indicate that populations carry different variants of the ACE2 gene,” said Maarit Tiirikainen, an associate professor at UH Mānoa, who is partnering on the study.
This variation in the gene coding for the ACE2 receptor may have an effect on the number of ACE2 receptors on the lung cells, as well as on how effectively the virus binds to the receptor. There may also be genetic differences in immunity and other important genes explaining why some people get more sick than others.
“Based on genetics, certain individuals and populations may be impacted more severely,” said Cyril Moukarzel, CEO of LifeDNA, a partner in the University of Hawai’i study.
Susceptibility to the coronavirus has been found to be negatively associated with having a genetic propensity to absorb Vitamin C. Africans are genetically good at absorbing Vitamin C. Across Africa, roughly 50% of people carry the Vitamin C-friendly variant and in some African countries, it is as high as 70%. In the US, 41% of whites carry this variant, compared to 55% of blacks, and 52% of Hispanics, but only 31% of Asians.
There is at least one gene that might increase the likelihood of blacks and other population groups of contracting or suffering serious health consequences from Covid-19. The G6PD gene provides instructions for making an enzyme, active in all cells. It impacts the normal processing of carbohydrates and helps to protect red blood cells from damage and premature destruction. In a series of studies since 2007, Chinese researchers reported an impaired immune system response to human coronavirus 229E in G6PD. The deficiency is most prevalent in some Asian and Mediterranean populations.

There is even fragmentary population based data related to susceptibility to various coronaviruses. Rare mutations are believed to have helped insulate southern Asians from severe acute reparatory syndrome (SARS). And contradicting racist early 20th century theories about African Americans’ increased susceptibility to disease and reduced access to health care, studies have shown that during the 1918 epidemic the incidence of influenza was significantly lower in African Americans.
Why were blacks less susceptible? According to one 2016 study of influenza vaccines, when exposed to flu, “African Americans mounted higher virus neutralizing and IgG antibody responses to the H1N1 component of IIV3 or 4 compared to Caucasians.
The story of how Covid-19 impacts different racial groups in the US is yet to be written. Those of African-ancestry are  certainly not insulated from the ravages of the disease. Although few regions in the US are compiling data by race, Milwaukee is. African Americans made up almost half of the county’s 945 cases and 81% of its 27 deaths, as of April 3, in a county whose population is 26% black. In Michigan, 14% black, African Americans made up 35% of cases and 40% of deaths. The concentration of the African-American population in many hard-hit cities explains some of these disparities.
certainly not insulated from the ravages of the disease. Although few regions in the US are compiling data by race, Milwaukee is. African Americans made up almost half of the county’s 945 cases and 81% of its 27 deaths, as of April 3, in a county whose population is 26% black. In Michigan, 14% black, African Americans made up 35% of cases and 40% of deaths. The concentration of the African-American population in many hard-hit cities explains some of these disparities.
Much of the differences also could be accounted for by health disparities between blacks and whites in the US or access to advanced health care during this crisis. The percentages also are based on a limited number of reported cases. Much more data are necessary to draw conclusions about racial disparities, and to tease out cultural and genetic factors.
What does all this add up to? It’s complicated. There is intriguing but hardly definitive evidence to suggest that the immune system of populations in sub-Saharan Africa might have evolved to be better at fighting off influenza than the white immune system. Research needs to be done and will be done, and appropriately. Differences in treatment response or disease prevalence between racial/ethnic groups should be studied, carefully, while avoiding naive inferences about genetic causation. Equally, gratuitous dismissal of a genetic interpretation without evidence for doing so is also unjustified.
Of course, there is an understandable fear that such research into human genetic diversity may revive the egregious racist beliefs that so blighted the world for centuries, and that still bubble beneath the surface in many modern societies. At the same time, however, the insights from population-based research may prove invaluable in combating the very diseases that so heavily impact marginalized or less affluent peoples.
Time may yet prove wrong this speculation about possible limited immunity by some African populations to Covid-19 (though we can perhaps still pray that the continent is spared the catastrophe of disease and death now afflicting other areas of the globe). But that is beside the point.
Africa’s vast reservoir of genetic diversity could reveal much about our species’ long and deadly relationship with disease. And in a time of truly global crisis, such knowledge might help protect the whole of humanity.
Patrick Whittle has a PhD in philosophy and is a freelance writer with a particular interest in the social and political implications of modern biological science. Follow him on his website patrickmichaelwhittle.com or on Twitter @WhittlePM
Jon Entine is executive director of the Genetic Literacy Project. BIO. Follow him on Twitter @JonEntine