The Food and Drug Administration on Thursday approved a new test for coronavirus antibodies, the first for use in the United States.
Currently available tests are designed to find fragments of viral genes indicating an ongoing infection. Doctors swab the nose and throat, and amplify any genetic material from the virus found there.
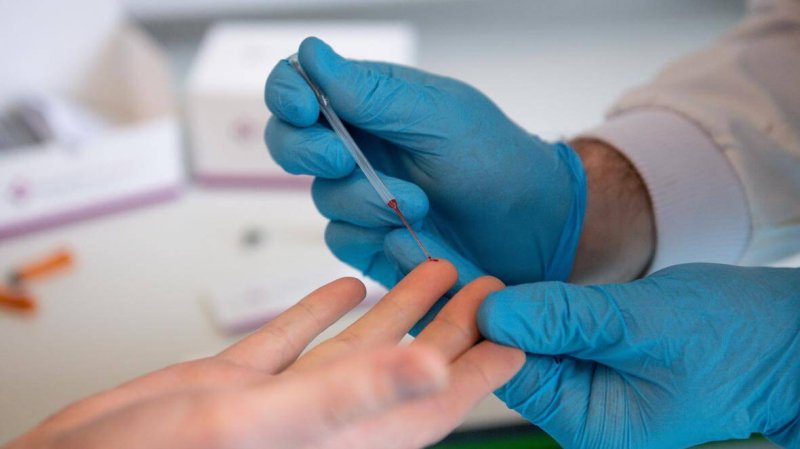
The new test, by contrast, looks for protective antibodies in a finger prick of blood. It tells doctors whether a patient has ever been exposed to the virus and now may have some immunity.
That is important for several reasons. People with immunity might be able to venture safely from their homes and help shore up the work force. It may be particularly important for doctors and nurses to know whether they have antibodies.
…
The test delivers results in about 15 minutes. But just having antibodies does not guarantee immunity from the coronavirus.
The new test looks only for the presence of the antibodies and delivers a qualitative yes or no answer — it does not say how well any antibodies are working, said Dr. Angela Rasmussen, a virologist at Columbia University in New York.